1. Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019; 40:87–165. PMID:
30165437.
2. Dehmer GJ, Weaver D, Roe MT, et al. A contemporary view of diagnostic cardiac catheterization and percutaneous coronary intervention in the United States: a report from the CathPCI Registry of the National Cardiovascular Data Registry, 2010 through June 2011. J Am Coll Cardiol. 2012; 60:2017–2031. PMID:
23083784.
3. Toth GG, Toth B, Johnson NP, et al. Revascularization decisions in patients with stable angina and intermediate lesions: results of the international survey on interventional strategy. Circ Cardiovasc Interv. 2014; 7:751–759. PMID:
25336468.
4. Tu S, Barbato E, Köszegi Z, et al. Fractional flow reserve calculation from 3-dimensional quantitative coronary angiography and TIMI frame count: a fast computer model to quantify the functional significance of moderately obstructed coronary arteries. JACC Cardiovasc Interv. 2014; 7:768–777. PMID:
25060020.
5. Tu S, Westra J, Yang J, et al. Diagnostic accuracy of fast computational approaches to derive fractional flow reserve from diagnostic coronary angiography: the international multicenter FAVOR pilot study. JACC Cardiovasc Interv. 2016; 9:2024–2035. PMID:
27712739.
6. Xu B, Tu S, Qiao S, et al. Diagnostic accuracy of angiography-based quantitative flow ratio measurements for online assessment of coronary stenosis. J Am Coll Cardiol. 2017; 70:3077–3087. PMID:
29101020.
7. Collet C, Onuma Y, Sonck J, et al. Diagnostic performance of angiography-derived fractional flow reserve: a systematic review and Bayesian meta-analysis. Eur Heart J. 2018; 39:3314–3321. PMID:
30137305.

8. Westra J, Andersen BK, Campo G, et al. Diagnostic performance of in-procedure angiography-derived quantitative flow reserve compared to pressure-derived fractional flow reserve: the FAVOR II Europe-Japan study. J Am Heart Assoc. 2018; 7:e009603. PMID:
29980523.

9. Westra J, Tu S, Winther S, et al. Evaluation of coronary artery stenosis by quantitative flow ratio during invasive coronary angiography: the WIFI II study (Wire-Free Functional Imaging II). Circ Cardiovasc Imaging. 2018; 11:e007107. PMID:
29555835.

10. Hwang D, Choi KH, Lee JM, et al. Diagnostic agreement of quantitative flow ratio with fractional flow reserve and instantaneous wave-free ratio. J Am Heart Assoc. 2019; 8:e011605. PMID:
30977410.

11. Emori H, Kubo T, Kameyama T, et al. Quantitative flow ratio and instantaneous wave-free ratio for the assessment of the functional severity of intermediate coronary artery stenosis. Coron Artery Dis. 2018; 29:611–617. PMID:
29965837.

12. Hwang D, Jeon KH, Lee JM, et al. Diagnostic performance of resting and hyperemic invasive physiological indices to define myocardial ischemia: validation with
13N-ammonia positron emission tomography. JACC Cardiovasc Interv. 2017; 10:751–760. PMID:
28365268.
13. Lee JM, Hwang D, Park J, et al. Exploring coronary circulatory response to stenosis and its association with invasive physiologic indexes using absolute myocardial blood flow and coronary pressure. Circulation. 2017; 136:1798–1808. PMID:
28851731.

14. Lee JM, Kim CH, Koo BK, et al. Integrated myocardial perfusion imaging diagnostics improve detection of functionally significant coronary artery stenosis by
13N-ammonia positron emission tomography. Circ Cardiovasc Imaging. 2016; 9:e004768. PMID:
27609817.

15. Gould KL, Johnson NP, Bateman TM, et al. Anatomic versus physiologic assessment of coronary artery disease. Role of coronary flow reserve, fractional flow reserve, and positron emission tomography imaging in revascularization decision-making. J Am Coll Cardiol. 2013; 62:1639–1653. PMID:
23954338.
16. Stuijfzand WJ, Uusitalo V, Kero T, et al. Relative flow reserve derived from quantitative perfusion imaging may not outperform stress myocardial blood flow for identification of hemodynamically significant coronary artery disease. Circ Cardiovasc Imaging. 2015; 8:e002400. PMID:
25596142.

17. Johnson NP, Gould KL. Integrating noninvasive absolute flow, coronary flow reserve, and ischemic thresholds into a comprehensive map of physiological severity. JACC Cardiovasc Imaging. 2012; 5:430–440. PMID:
22498334.

18. Hajjiri MM, Leavitt MB, Zheng H, Spooner AE, Fischman AJ, Gewirtz H. Comparison of positron emission tomography measurement of adenosine-stimulated absolute myocardial blood flow versus relative myocardial tracer content for physiological assessment of coronary artery stenosis severity and location. JACC Cardiovasc Imaging. 2009; 2:751–758. PMID:
19520347.

19. Sen S, Escaned J, Malik IS, et al. Development and validation of a new adenosine-independent index of stenosis severity from coronary wave-intensity analysis: results of the ADVISE (ADenosine Vasodilator Independent Stenosis Evaluation) study. J Am Coll Cardiol. 2012; 59:1392–1402. PMID:
22154731.
20. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988; 44:837–845. PMID:
3203132.

21. Johnson NP, Koo BK. Coronary psychology: do you believe? JACC Cardiovasc Interv. 2018; 11:1492–1494. PMID:
30093054.
22. Lee HS, Lee JM, Nam CW, et al. Consensus document for invasive coronary physiologic assessment in Asia-Pacific countries. Cardiol J. 2019; 26:215–225. PMID:
31225632.

23. Adjedj J, Xaplanteris P, Toth G, et al. Visual and quantitative assessment of coronary stenoses at angiography versus fractional flow reserve: the impact of risk factors. Circ Cardiovasc Imaging. 2017; 10:e006243. PMID:
28687539.

24. Toth G, Hamilos M, Pyxaras S, et al. Evolving concepts of angiogram: fractional flow reserve discordances in 4000 coronary stenoses. Eur Heart J. 2014; 35:2831–2838. PMID:
24644308.

25. Mejía-Rentería H, Lee JM, Lauri F, et al. Influence of microcirculatory dysfunction on angiography-based functional assessment of coronary stenoses. JACC Cardiovasc Interv. 2018; 11:741–753. PMID:
29673505.

26. Johnson NP, Kirkeeide RL, Gould KL. Is discordance of coronary flow reserve and fractional flow reserve due to methodology or clinically relevant coronary pathophysiology? JACC Cardiovasc Imaging. 2012; 5:193–202. PMID:
22340827.

27. Johnson NP, Kirkeeide RL, Gould KL. Coronary anatomy to predict physiology: fundamental limits. Circ Cardiovasc Imaging. 2013; 6:817–832. PMID:
24046379.
28. Chu M, Dai N, Yang J, Westra J, Tu S. A systematic review of imaging anatomy in predicting functional significance of coronary stenoses determined by fractional flow reserve. Int J Cardiovasc Imaging. 2017; 33:975–990. PMID:
28265791.

29. Tahari AK, Lee A, Rajaram M, et al. Absolute myocardial flow quantification with
82Rb PET/CT: comparison of different software packages and methods. Eur J Nucl Med Mol Imaging. 2014; 41:126–135. PMID:
23982454.
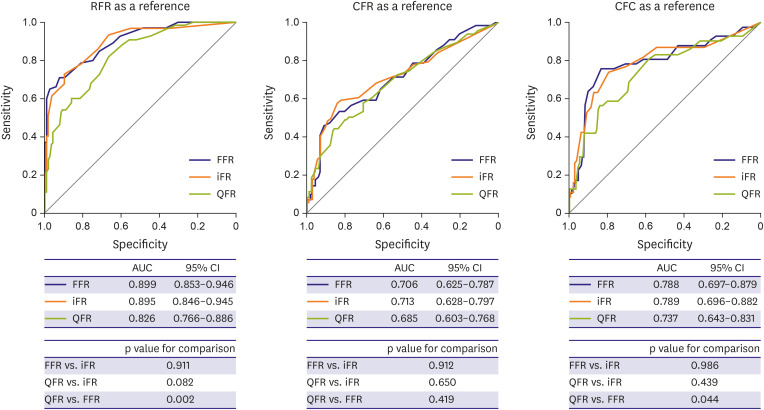
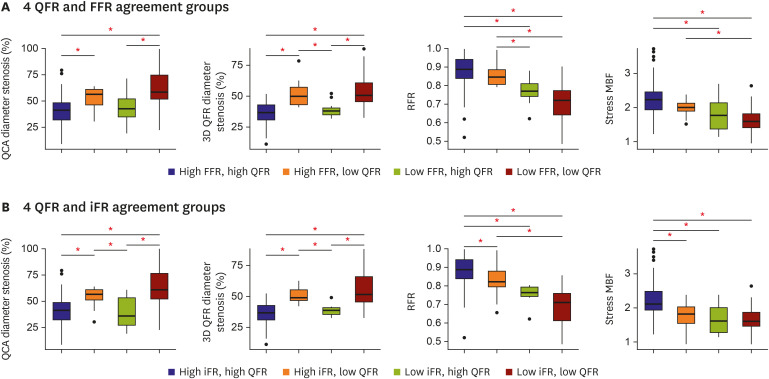
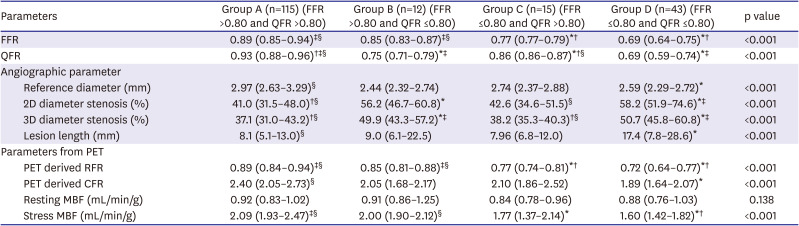
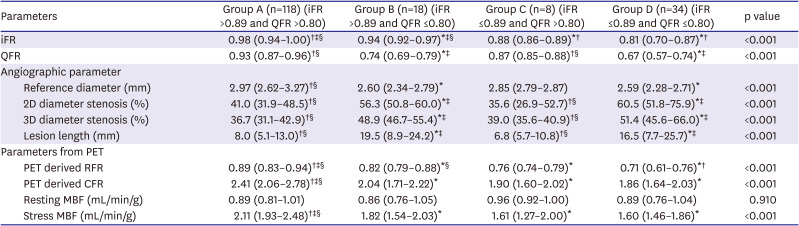




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download