1. Birch J, Kolle RU, Kunkel M, Paulus W, Upadhyay P. Acquired colour deficiency in patients with Parkinson's disease. Vision Res. 1998; 38(21):3421–3426. PMID:
9893859.

2. Matsui H, Udaka F, Tamura A, Oda M, Kubori T, Nishinaka K, et al. Impaired visual acuity as a risk factor for visual hallucinations in Parkinson's disease. J Geriatr Psychiatry Neurol. 2006; 19(1):36–40. PMID:
16449759.


3. Djamgoz MB, Hankins MW, Hirano J, Archer SN. Neurobiology of retinal dopamine in relation to degenerative states of the tissue. Vision Res. 1997; 37(24):3509–3529. PMID:
9425527.


4. Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, et al. Optical coherence tomography. Science. 1991; 254(5035):1178–1181. PMID:
1957169.



5. Inzelberg R, Ramirez JA, Nisipeanu P, Ophir A. Retinal nerve fiber layer thinning in Parkinson disease. Vision Res. 2004; 44(24):2793–2797. PMID:
15342223.


6. Aaker GD, Myung JS, Ehrlich JR, Mohammed M, Henchcliffe C, Kiss S. Detection of retinal changes in Parkinson's disease with spectral-domain optical coherence tomography. Clin Ophthalmol. 2010; 4:1427–1432. PMID:
21188154.


7. Altintaş O, Işeri P, Özkan B, Cağlar Y. Correlation between retinal morphological and functional findings and clinical severity in Parkinson's disease. Doc Ophthalmol. 2008; 116(2):137–146. PMID:
17962989.


8. Albrecht P, Müller AK, Südmeyer M, Ferrea S, Ringelstein M, Cohn E, et al. Optical coherence tomography in parkinsonian syndromes. PLoS One. 2012; 7(4):e34891. PMID:
22514688.

9. Roth NM, Saidha S, Zimmermann H, Brandt AU, Isensee J, Benkhellouf-Rutkowska A, et al. Photoreceptor layer thinning in idiopathic Parkinson's disease. Mov Disord. 2014; 29(9):1163–1170. PMID:
24789530.


10. Tsironi EE, Dastiridou A, Katsanos A, Dardiotis E, Veliki S, Patramani G, et al. Perimetric and retinal nerve fiber layer findings in patients with Parkinson's disease. BMC Ophthalmol. 2012; 12(1):54. PMID:
23031247.



11. Hoehn MM, Yahr MD. Parkinsonism: onset, progression, and mortality. 1967. Neurology. 1998; 50(2):318. PMID:
9484345.

12. Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992; 55(3):181–184. PMID:
1564476.



13. Martinu K, Nagano-Saito A, Fogel S, Monchi O. Asymmetrical effect of levodopa on the neural activity of motor regions in PD. PLoS One. 2014; 9(11):e111600. PMID:
25369523.

14. Tanner JJ, Levy SA, Schwab NA, Hizel LP, Nguyen PT, Okun MS, et al. Marked brain asymmetry with intact cognitive functioning in idiopathic Parkinson's disease: a longitudinal analysis. Clin Neuropsychol. 2017; 31(3):654–675. PMID:
27813459.


15. Marsden CD, Parkes JD, Quinn N. 7 - Fluctuations of disability in Parkinson's disease – clinical aspects. Mov Disord. 1981; 96–122.
16. Altemir I, Oros D, Elía N, Polo V, Larrosa JM, Pueyo V. Retinal asymmetry in children measured with optical coherence tomography. Am J Ophthalmol. 2013; 156(6):1238–1243.e1. PMID:
24075424.


17. Yang M, Wang W, Xu Q, Tan S, Wei S. Interocular symmetry of the peripapillary choroidal thickness and retinal nerve fibre layer thickness in healthy adults with isometropia. BMC Ophthalmol. 2016; 16(1):182. PMID:
27756260.



18. Lin PW, Chang HW, Lai IC, Tsai JC, Poon YC. Intraocular retinal thickness asymmetry in early stage of primary open angle glaucoma and normal tension glaucoma. Int J Ophthalmol. 2018; 11(8):1342–1351. PMID:
30140639.



19. Shreve LA, Velisar A, Malekmohammadi M, Koop MM, Trager M, Quinn EJ, et al. Subthalamic oscillations and phase amplitude coupling are greater in the more affected hemisphere in Parkinson's disease. Clin Neurophysiol. 2017; 128(1):128–137. PMID:
27889627.


20. Plotnik M, Giladi N, Balash Y, Peretz C, Hausdorff JM. Is freezing of gait in Parkinson's disease related to asymmetric motor function? Ann Neurol. 2005; 57(5):656–663. PMID:
15852404.


21. Uitti RJ, Baba Y, Whaley NR, Wszolek ZK, Putzke JD. Parkinson disease: handedness predicts asymmetry. Neurology. 2005; 64(11):1925–1930. PMID:
15955945.


22. Ham JH, Lee JJ, Kim JS, Lee PH, Sohn YH. Is dominant-side onset associated with a better motor compensation in Parkinson's disease? Mov Disord. 2015; 30(14):1921–1925. PMID:
26408124.


23. Huang P, Tan YY, Liu DQ, Herzallah MM, Lapidow E, Wang Y, et al. Motor-symptom laterality affects acquisition in Parkinson's disease: a cognitive and functional magnetic resonance imaging study. Mov Disord. 2017; 32(7):1047–1055. PMID:
28712121.


24. Prasad S, Saini J, Yadav R, Pal PK. Motor asymmetry and neuromelanin imaging: concordance in Parkinson's disease. Parkinsonism Relat Disord. 2018; 53:28–32. PMID:
29709506.


25. Mendoza-Santiesteban CE, Palma JA, Ortuño-Lizarán I, Cuenca N, Kaufmann H. Pathologic confirmation of retinal ganglion cell loss in multiple system atrophy. Neurology. 2017; 88(23):2233–2235. PMID:
28490649.



26. Sari ES, Koc R, Yazici A, Sahin G, Ermis SS. Ganglion cell-inner plexiform layer thickness in patients with Parkinson disease and association with disease severity and duration. J Neuroophthalmol. 2015; 35(2):117–121. PMID:
25485861.


27. Ahn J, Lee JY, Kim TW, Yoon EJ, Oh S, Kim YK, et al. Retinal thinning associates with nigral dopaminergic loss in de novo Parkinson disease. Neurology. 2018; 91(11):e1003–12. PMID:
30111550.

28. Jiménez B, Ascaso FJ, Cristóbal JA, López del Val J. Development of a prediction formula of Parkinson disease severity by optical coherence tomography. Mov Disord. 2014; 29(1):68–74. PMID:
24458320.


29. Satue M, Garcia-Martin E, Fuertes I, Otin S, Alarcia R, Herrero R, et al. Use of Fourier-domain OCT to detect retinal nerve fiber layer degeneration in Parkinson's disease patients. Eye (Lond). 2013; 27(4):507–514. PMID:
23429414.



30. Yust-Katz S, Tesler D, Treves TA, Melamed E, Djaldetti R. Handedness as a predictor of side of onset of Parkinson's disease. Parkinsonism Relat Disord. 2008; 14(8):633–635. PMID:
18346926.


31. Archibald NK, Clarke MP, Mosimann UP, Burn DJ. Retinal thickness in Parkinson's disease. Parkinsonism Relat Disord. 2011; 17(6):431–436. PMID:
21454118.

32. Hajee ME, March WF, Lazzaro DR, Wolintz AH, Shrier EM, Glazman S, et al. Inner retinal layer thinning in Parkinson disease. Arch Ophthalmol. 2009; 127(6):737–741. PMID:
19506190.


33. Mailankody P, Battu R, Khanna A, Lenka A, Yadav R, Pal PK. Optical coherence tomography as a tool to evaluate retinal changes in Parkinson's disease. Parkinsonism Relat Disord. 2015; 21(10):1164–1169. PMID:
26297381.


34. Garcia-Martin E, Rodriguez-Mena D, Satue M, Almarcegui C, Dolz I, Alarcia R, et al. Electrophysiology and optical coherence tomography to evaluate Parkinson disease severity. Invest Ophthalmol Vis Sci. 2014; 55(2):696–705. PMID:
24425856.


35. Bohnen NI, Minoshima S, Giordani B, Frey KA, Kuhl DE. Motor correlates of occipital glucose hypometabolism in Parkinson's disease without dementia. Neurology. 1999; 52(3):541–546. PMID:
10025784.


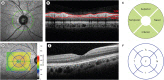
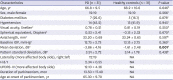
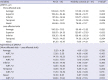

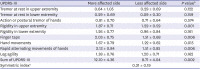






 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download