Maternal serum screening
Recommendation: Pregnant women should be informed of common fetal aneuploidy that can be detected, risks for chromosomal abnormality according to the maternal age, DR, and false-positive rate (FPR) for common fetal aneuploidy with each screening test, limitations, as well as the benefits and risks of invasive diagnostic testing (Level 2++, Grade A).
Summary of evidence: Chromosomal abnormality in which a chromosome is either missing or additionally present is known as aneuploidy. Autosomal trisomies, such as DS, are the most common, and their incidence rises proportionately to maternal age. While maternal serum screening during pregnancy can detect trisomy 21, 18, and 13, it could not screen for all chromosomal abnormalities. Maternal serum screening calculates the risk for aneuploidy by multiplying the risk for aneuploidy according to the maternal age by the likelihood ratio (LR) for aneuploidy based on serum screening test results. The cutoff for high-risk is 1:270, which is the risk for fetal DS for a woman at age 35 or above.
3
Maternal serum screening is classified into first trimester screening and second trimester screening. The first trimester screening measures two serum biomarkers — pregnancy-associated plasma protein A (PAPP-A) and free beta-human chorionic gonadotropin (hCG), and NT at 11–136/7 weeks of gestation and it has a DR for DS of 80–84% with a 5% FPR and positive predictive value (PPV) of 3%–4%. The second trimester screening measures four serum biomarkers—alpha-fetoprotein (AFP), hCG, unconjugated estriol, and inhibin A. The triple screening, which was first introduced in the 1990s, measures three biomarkers (AFP, hCG, and unconjugated estriol) in the second trimester, and its DR for DS was about 69% with a 5% FPR. The DR for DS was improved to 81% with a 5% FPR in the subsequently developed quad screening, which measures inhibin-A in addition to the previous three biomarkers.
In recent years, integrated screening test and sequential screening test, which combines the first trimester and second trimester maternal serum screening tests, are performed. Fully integrated screening test measures NT and PAPP-A between 11 and 136/7 weeks of gestation and performs quad screening test between 15 and 216/7 weeks' gestation. It has the highest DR for DS at 94%–96% (5% FPR) and PPV of 5%. Serum integrated screening test, which excludes NT, has a lower DS DR of 85%–88% compared with fully integrated test. Sequential test involves two types of sequential test: stepwise sequential test and contingent sequential test. First, maternal serum screening was examined in the first trimester to identify high-risk women with reference to a risk cutoff of 1:30 and these high-risk women undergo invasive diagnostic testing in the first trimester. With the stepwise sequential test, all women who were not identified to be at high risk in the first trimester maternal serum screening undergo the quad screening in the second trimester. With the contingent sequential test, quad screening in the second trimester is performed only on the intermediate risk group (1:30–1:1,500) for DS in the first trimester maternal serum screening and omitted in the low- risk group (< 1:1,500). DR for DS is 92% (5.1% FPR) for the stepwise sequential test and 91% (4.5% FPR) for the contingent sequential test. Whereas maternal serum screening enables noninvasive screening of fetuses with elevated risks for trisomy 21, 18, and 13, it cannot detect all chromosomal abnormalities. In other words, being identified as low-risk in the maternal serum screening simply means that these women have a low-risk for the detectable chromosomal abnormalities and not that their chromosomes are all normal. While integrated test has the highest DR for DS among maternal serum screening tests, it has some limitations including not presenting the results of the first trimester screening, having to complete the second trimester screening, and having to undergo both blood samples in a same facility, which may raise issues pertaining to patient adherence. During the counseling for maternal serum screening for fetal aneuploidy, the healthcare providers must also inform women of the benefits and risks of invasive diagnostic testing. One benefit of invasive diagnostic testing, namely CVS and amniocentesis, is that they reveal the numeral and structural anomalies of all chromosomes, but they have a risk for fetal loss. The rate of fetal loss prior to 24 weeks' gestation due to amniocentesis between 15 and 22 weeks of gestation was reported to be 0.13%, and CVS in the first trimester was higher at about 1.2%. Counseling about the limitations of maternal serum screening should be recorded, and the women's decision should be fully respected.
Recommendation: It is ideal to give counseling at the first prenatal visit, and counseling is recommended to be given early in pregnancy (Level 2++, Grade A).
Summary of evidence: Women should be given sufficient time to understand the information about fetal aneuploidy screening and invasive diagnostic testing offered during counseling and make their own informed decision. In the first trimester, serum screening would be performed in addition to NT measurement at 11–13
6/7 weeks, and it would be ideal to give counseling about fetal aneuploidy screening and invasive diagnostic testing before the antenatal visit for NT measurement, so that fetal aneuploidy screening could be performed at the appropriate timing and women can make their own choices. It has been reported that 96.8% of women who receive prenatal care within the first trimester choose integrated screening, which has the highest DR for DS among maternal serum screening tests. American College of Obstetrics & Gynecology recommends offering fetal aneuploidy screening and invasive diagnostic testing on the first prenatal visit, because women have fewer prenatal visits overall due to differences in access to healthcare and insurance systems.
15 However, considering the high frequency of miscarriage in early pregnancy, the National Health Insurance system, and relatively high access to healthcare in Korea, it would be more appropriate to set the timing of counseling as any time before the NT measurement in the first trimester instead of at the first prenatal visit.
Recommendation: All pregnant women should be informed about maternal serum screening regardless of their age (Level 2++, Grade A).
Summary of evidence: As the incidence of fetal aneuploidy increases with increased maternal age, advanced maternal age at 35 years or older itself was considered an indication for invasive prenatal testing in the past.
3 Since then, maternal age continued to rise and advances of first trimester maternal serum screening and NT have enhanced the accuracy of maternal serum screening. These days, because Korea features a high percentage of advanced maternal age at 35 years or older, at 33.3% in 2019, one in every three women must undergo amniocentesis if amniocentesis were to be recommended to all pregnant women aged 35 years or older. According to Resta et al. the percentage of advanced maternal age at or above 35 years rose from 4.57% in 1980 to 14% in 2002. If amniocentesis would be recommended for all pregnant women aged 35 years or older, it detects 51% of DS with 13.77% FPR, showing an inferior screening power compared to the maternal serum screening at the time. With improved accuracy of maternal serum screening and increased advanced maternal age, assessing the risk for DS with maternal serum screening is superior to assessing it based on maternal age, and for this reason, invasive diagnostic testing is not primarily recommended solely based on maternal age.
The risk for fetal aneuploidy in twin pregnancy differs not only by maternal age but also by zygosity. Dizygotic twins are at an elevated risk for fetal DS compared to single fetuses of women at the same maternal age, and thus the risk for aneuploidy is comparable between twin pregnancy aged 32 years or older and singleton pregnancy aged 35 years older. In recent years, the increased use of assisted reproduction techniques (ARTs) have led to an increase in twin pregnancies, and the incidence of both dizygotic twins and monozygotic twins have risen. Zygosity may be diagnosed based on the use of ART or chorionicity, but chorionicity alone is not sufficient to accurately determine zygosity, as 33% of monozygotic twins are dichorionic. Moreover, because the risk of fetal loss due to invasive diagnostic testing was known to be higher for twins than for singletons, assessing fetal risk for DS using maternal serum screening is recommended over using maternal age alone in twin pregnancies as well. Performing invasive diagnostic testing without screening should be limited to cases involving intrauterine fetal death or structural abnormality of one fetus.
Recommendation: If maternal serum screening results are low-risk, performing additional independent serum screening is not recommended, as it may increase false positive result (Level 2++, Grade A).
Summary of evidence: Performing first trimester and second trimester serum screening independently of one another without using them as a part of combined test increases FPR without increasing the DR for fetal aneuploidy. Because the quad screening has a lower DR for fetal aneuploidy with higher FPR compared to the first trimester maternal serum screening including NT, first trimester screening is superior to the quad screening when performed independently. Therefore, both healthcare providers and pregnant women must be aware of the fact that the risk for FPR may increase without better DR for fetal aneuploidy if a woman who was low-risk in the first trimester maternal serum screening undergoes the quad screening in the second trimester at a different facility.
Recommendation: Healthcare providers should explain that the result indicating high-risk does not confirm fetal abnormality and offer counseling about cfDNA screening or invasive diagnostic testing (Level 2++, Grade A).
Summary of evidence: In the maternal serum screening, the individual risks for trisomy 21 and 18 are analyzed, and the woman is classified as high-risk if a certain threshold of risk is met. It should be explained to the woman that the result indicating high-risk does not confirm fetal abnormality and that it is an outcome of a combined analysis of the existing risk for fetal aneuploidy according to maternal age and the serum test results. Further, after informing the woman of the accuracy of each serum screening test and confirming that they have understood the test results, healthcare providers should explain the pros and cons of further tests to be considered in detail.
Healthcare providers should first recommend invasive diagnostic testing to high-risk women and explain the method, safety, and cost. Women should be informed that CVS could be performed prior to 14 weeks' gestation and amniocentesis could be performed after 14 weeks' gestation and should undergo testing accordingly. CVS can detect chromosomal abnormality at a rate of 98%–99%, with < 1% mosaicism, < 1% risk of maternal cell contamination, and 0.5%–1.2% procedure-related fetal loss rate. The accuracy of genetic amniocentesis performed in the second and third trimesters was reported as 99.8%–99.9%, and while the procedure-related fetal loss rate was 1/200, that is, 0.5%, but recent reports showed lower fetal loss rate at 0.06%–0.13%. Amniocentesis is not recommended in the first trimester due to increased risk of amniotic fluid leakage and equinovarus.
16
While fetal chromosomal abnormality can be diagnosed only based on invasive diagnostic testing, some women prefer noninvasive ‘blood’ test as an alternative due to concerns about fetal safety from invasive testing. Maternal serum screening should not be performed again in these women, as doing so would be not cost-effective. The cfDNA screening detects trisomy 21, 18, and 13 at a high DR with low FPR by analyzing the amount of fetal DNA circulating in maternal plasma and thus can be a reasonable alternative for women who prefer a noninvasive test. The cfDNA screening has been showing a highest sensitivity of 99.3% and specificity of 99.8% for trisomy 21, 97.4% and 99.8% for trisomy 18. The sensitivity is 91% and specificity is > 99% for trisomy 13 and monosomy X.
1718 However, the risk for fetal chromosomal abnormality other than common autosomal trisomies was reported to be 2% in cases with high-risk in the traditional maternal serum screening and low-risk in the cfDNA screening. Also, women should be well informed that the cfDNA screening could delay the diagnosis for fetal chromosomal abnormality in true positive cases because women with high-risk in the cfDNA screening must be taken invasive diagnostic testing. Women should be well informed that cfDNA screening is not a diagnostic test.
Recommendation: Clinical information such as gestational age, maternal body weight, race, insulin-dependent diabetes mellitus, number of fetuses, and ultrasonographic fetal size should be provided to the laboratory (Level 2++, Grade A).
Summary of evidence: Various clinical factors are involved in the analysis of the results of maternal serum screening. Thus, this clinical information must be accurately recorded and included in the analysis in order to obtain accurate results. The concentrations of each serum biomarkers are converted to multiples of median (MoM) according to gestational age. Calculating the gestational age based on ultrasonographic measurement has been reported to lower the FPR by about 2%. With increasing maternal body weight, blood volume increases and dilutes the concentration of maternal serum biomarkers, thereby reducing the concentration of maternal serum factors. Adjusting for maternal body weight during the second trimester screening could increase the DR by 1% with a fixed FPR or reduce the FPR by about 0.2% with a fixed DR. The mean values of serum biomarkers vary across ethnicities. Compared to Caucasian women, South Asian women have 6% lower AFP, 7% higher unconjugated estriol, 6% higher hCG, and 17% higher PAPP-A, and black women have 15% higher AFP, 18% lower hCG, 8% higher inhibin A, and 35% higher PAPP-A. Therefore, adjusting for racial differences may enhance the DR at a fixed FPR. The concentrations of two second trimester serum biomarkers—AFP and unconjugated estriol—are 10% and 5% lower, respectively, in insulin-dependent diabetes mellitus, while the concentrations of other biomarkers do not vary in relation to diabetes mellitus. Women who conceived with ART show high second trimester free beta-hCG and total hCG, and studies have reported that the FPR may increase by two times in these women. While Wald et al. proposed that high FPR could be avoided by adjusting for the use of ART,
19 recent studies stated that adjusting for ART does not improve the FPR. First trimester PAPP-A concentration is low in ART pregnancies, but hCG results are inconsistent.
Recommendation: For women who underwent the first trimester screening, maternal serum AFP is recommended for screening of NTD in the second trimester and then ultrasonography is recommended for detection of other structural abnormality. For women above 20 weeks' gestation, ultrasonography should be performed for screening of NTD (Level 2++, Grade A).
Summary of evidence: NTD screening should be recommended to all pregnant women regardless of age. MSAFP can be performed between 15 and 216/7 weeks of gestation, desirably between 16 and 18 weeks for NTD screening, and analysis is difficult prior to 15 weeks. Because the risk for NTD cannot be predicted with the first trimester maternal serum screening or cell-free DNA screening, MSAFP must be performed in the second trimester in such cases. NTD is a different congenital abnormality from fetal aneuploidy, so healthcare providers should give information about MSAFP screening to women prior to 216/7 weeks of gestation even if they decided not to undergo the fetal aneuploidy screening. If MSAFP screening is chosen, the results must be analyzed after adjusting for ultrasound-based gestation age, body weight, insulin-dependent diabetes mellitus, race, and a number of fetuses. Using a cutoff of 2.5 MoM for MSAFP in the second trimester, 73%–83% of open NTD was diagnosed.
Recommendation: Invasive diagnostic testing for fetal chromosomal abnormality and detailed ultrasonography to examine structural anomalies should be performed (Level 2++, Grade A).
Summary of evidence: NT can be measured between 11 and 136/7 weeks of gestation, with the highest accuracy between 11 and 12 weeks. Chromosomal abnormality was found in 35% of cases with NT between 3–8 mm, and various structural abnormalities were associated although fetal karyotype was normal. NT increases by 15%–20% depending on gestational age, so recent DS screening programs calculate the risk by converting the values into MoM for the specific gestational age. According to a single center study about gestational age and NT thickness in Korea by Chung et al. in 2004, the ethnic difference was not significant, and as no past studies have reported a racial difference in NT, it would be acceptable to directly apply foreign criteria. If NT is ≥ 99th percentile or 3 mm or greater between 11 and 136/7 weeks of gestation regardless of gestational age, the likelihood of aneuploidy is high at 1:6, and even if combined with maternal serum screening, only 8% of these cases have the risk for DS lowered to 1:200. Most of these cases still are at high-risk and need to consider invasive diagnostic testing, based on which it would be appropriate to recommend invasive prenatal testing without serum screening. Moreover, enlarged NT to 3 mm or higher was only observed in 0.5% of the cases, so the overall medical cost would not increase much. An enlarged NT (≥ 3.0 mm) can detect fetal DS with a 69%–75% DR with 5.0%–8.1% FPR. Regarding cystic hygroma, 51% of the cases are accompanied by chromosomal abnormality, and 34% with normal karyotype are accompanied by major heart and skeletal anomalies. Therefore, when cystic hygroma is observed, clinicians should recommend invasive diagnostic testing and perform detailed ultrasonography to identify other structural anomalies.
Recommendation: Detailed ultrasonography for structural abnormality and fetal echocardiography should be performed in the second trimester and women should be informed of the possibility of genetic syndrome undetected through fetal karyotyping and increased risk for poor perinatal outcomes (Level 2++, Grade A).
Summary of evidence: Enlarged fetal NT is also associated with the genetic syndrome and fetal structural anomalies such as heart, abdominal wall, and musculoskeletal system in addition to fetal chromosomal abnormality. Survival rate decreases with increasing NT thickness, where survival rate decreases from 96.3% when NT < 3.5 mm to 44.4% when NT ≥ 6.5 mm, and the incidence of fetal abnormality and genetic syndrome also increase with increasing NT thickness. The incidence of cystic hygroma was reported to be 1/285 first-trimester pregnancies and of them, 51% have chromosome abnormality. Among cystic hygroma with normal karyotype, 34% had fetal structural abnormality and intrauterine death occurred in 8% of normal karyotype, and 4% of survivors were reported to have cerebral palsy and developmental disorder. Cystic hygroma has poorer prognosis compared to a simple enlargement of NT, where the risks for fetal aneuploidy, heart anomaly, and perinatal death are 5 times, 12 times, and 6 times higher, respectively. Therefore, if normal karyotype is confirmed in a fetus with enlarged NT or cystic hygroma in the first trimester, a detailed ultrasonography and fetal echocardiography should be performed in the second trimester to examine for structural anomalies. Clinicians should inform women with enlarged NT or cystic hygroma of the risk for undiagnosed genetic syndrome and risk for poor perinatal outcomes.
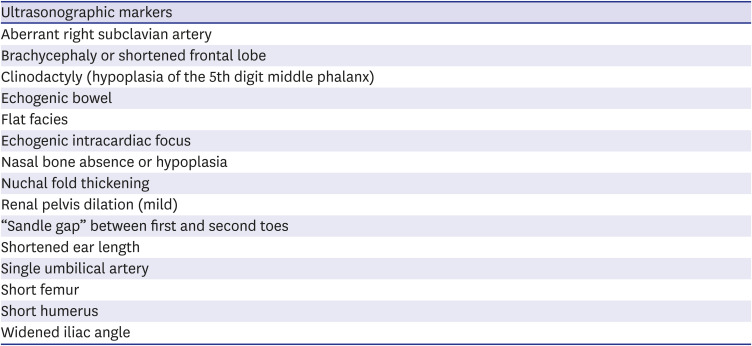
Recommendation: In cases with an isolated minor marker related to aneuploidy in the second trimester ultrasound, prenatal aneuploidy screening is recommended if it was not performed. If fetal aneuploidy screening was performed in the first or second trimester and the result was low-risk, an additional test is not recommended (Level 2++, Grade B).
Summary of evidence: Ultrasonographic minor markers are generally normal variants identified on second trimester but can be associated with fetal DS (
Table 3).
2021 They are relatively common enough to be found in 10% of normal pregnancies and do not mean fetal abnormalities or affect prognosis in absence of fetal aneuploidy. Because the LR of DS differs for each minor marker, each minor marker should be assessed in consideration of the woman's overall clinical situation. According to Nyberg et al.,
21 an analysis of the LR of nuchal fold thickening, echogenic bowel, short femur, echogenic intracardiac focus, and renal pelvis dilation for DS revealed that nuchal fold thickening had the highest LR (LR 11), followed by echogenic bowel (LR 6.7), with the remaining minor markers having a LR of 1.0–2.0. Other studies have identified nuchal fold thickening as the minor marker with the strongest association with DS, highlighting the need for detailed ultrasonography and counseling to evaluate for the presence of other minor markers in cases showing nuchal fold thickening in the second trimester ultrasound. One study reported that echogenic bowel could result from swallowing blood in the amniotic fluid and that it does not increase the risk for DS. Echogenic intracardiac focus is reported to occur in up to 30% of Asian pregnant women, which should be taken into consideration when applying it as a minor marker in Korean women. While nasal bone hypoplasia improves DR of DS when combined with other minor markers, additional test is not recommended solely based on nasal bone hypoplasia. Thus, if minor markers other than nuchal fold thickening are detected alone, it is desirable to perform fetal aneuploidy screening if it had not been previously performed and an additional test is not recommended if the fetal aneuploidy screening result was low-risk. When adjusting for the risk of existing maternal serum screening using minor markers, the relative LRs of positive and negative findings should be applied systematically, and if the marker is present alone, it has little impact on the existing maternal screening test. In particular, if cfDNA screening was already performed and its result was low-risk, the detection of a single minor marker does not warrant additional test or risk readjustment. Choroid plexus cyst was excluded from the list of minor markers of DS.
Table 3
Minor markers associated with fetal Down syndrome on second trimester
|
Ultrasonographic markers |
|
Aberrant right subclavian artery |
|
Brachycephaly or shortened frontal lobe |
|
Clinodactyly (hypoplasia of the 5th digit middle phalanx) |
|
Echogenic bowel |
|
Flat facies |
|
Echogenic intracardiac focus |
|
Nasal bone absence or hypoplasia |
|
Nuchal fold thickening |
|
Renal pelvis dilation (mild) |
|
“Sandle gap” between first and second toes |
|
Shortened ear length |
|
Single umbilical artery |
|
Short femur |
|
Short humerus |
|
Widened iliac angle |

Recommendation: Pregnant women should be informed that maternal serum screening tests have lower accuracy in twin pregnancies than in singleton pregnancies (Level 2++, Grade B).
Summary of evidence: The risk for fetal aneuploidy in twin pregnancy is affected by the number of fetuses and zygosity. When assessed according to maternal age, the risk for fetal DS is equal between monozygotic twin pregnancy and singleton pregnancy but increases in dizygotic twin pregnancy, as each of the dizygotic twin has a risk for fetal aneuploidy. However, data on maternal serum screening is relatively lacking for twin pregnancies compared to singleton pregnancies, and as maternal serum screening assesses the overall risk present in pregnancy as opposed to assessing the risk for each fetus, it has a lower accuracy for twin pregnancies compared to singleton pregnancies. According to the cohort study by Neveux et al.,
22 the DR for fetal DS with triple screening is 73% in monozygotic twin pregnancy, 43% in dizygotic twin pregnancy, and 53% overall with 5% FPR, which were lower than that in singleton pregnancy. According to Wald et al.'s study,
23 integrated test detected DS in 93% in monochorionic twins, 78% in dichorionic twins, and 80% in all twin pregnancies with 5% FPR, but the results were not confirmed with a prospective study. Based on the fact that integrated test that combines NT and maternal serum screening enhances the DR while lowering the FPR in singleton pregnancies, the same strategy is currently used for twin pregnancies; however, this method has not been prospectively validated, so pre- and post-test counseling is essential when performing maternal serum screening in twin pregnancy.
Recommendation: Maternal serum screening is not recommended when fetal demise or major structural abnormality is identified in one fetus in twin pregnancies. In such cases, invasive diagnostic testing can be considered to diagnose for fetal chromosomal abnormality (Level 2++, Grade B).
Summary of evidence: Multifetal pregnancy is associated with a higher incidence of miscarriage and fetal anomaly compared to a singleton pregnancy, and there are cases in which one fetus dies in utero or has structural abnormalities while the co-twin is normal. Vanishing twin, which refers to the loss of one embryo early in pregnancy, occurs in 10%–40% of all twin pregnancies, and this can impact the concentration of serum biomarkers even after a prolonged period. Concentrations of serum biomarkers were compared between vanishing twin and singleton pregnancy in consideration of same gestational age and maternal age, and the results showed that the vanishing twin group had 21% higher PAPP-A in the first trimester and 10% higher AFP and 13% higher inhibin A in the second trimester. The concentrations of PAPP-A and AFP levels showed a negative correlation with time interval between the occurrence of fetal demise and blood sampling, so PAPP-A value was similar to that of singleton pregnancy after 4 weeks, but it was found that the concentrations of hCG and inhibin A did not change over time. Therefore, maternal serum screening would lead confusion with its results in cases involving a vanishing twin and is not recommended. Similar to the consideration of invasive diagnostic testing in a singleton pregnancy with structural abnormality, it is appropriate to consider invasive diagnostic testing in twin pregnancies involving one fetus with structural abnormality.
Cell-free DNA screening
Recommendation: All pregnant women can be informed and offered the choice of cfDNA screening, although the first priority for these tests should be the high-risk group (Level 2++, Grade B).
Summary of evidence: The cfDNA screening shows similar sensitivity and specificity in both low and high-risk groups.
2425262728 Given the low PPV of this test in low-risk group and the possibility of ‘no call’ from inadequate sample, in terms of cost-effectiveness, it is not recommended for all pregnant women.
Recommendation: cfDNA screening can be used for the screening of trisomy 21, 18, 13 and sex-chromosome aneuploidy. It is not recommended for the screening of microdeletion (Level 2++, Grade B).
Summary of evidence: The cfDNA screening demonstrates high reliability in detecting trisomy 21, 18, and 13. While this method shows the highest sensitivity (99.3%) and specificity (99.8%) for trisomy 21, the sensitivity is 97.4% and specificity is 99.8% for trisomy 18. The sensitivity is 91% and specificity is > 99% for trisomy 13 and monosomy X. In the high-risk group, aged 40 or older, PPV is 87%, 68%, and 57% for trisomy 21, 18, and 13, respectively.
1718293031 The false positive rate of cfDNA screening for common trisomy is 1%, and even < 1% for trisomy 21. The performance of this screening may be affected by placental mosaicism, vanishing twin, maternal copy number variations and maternal malignant tumor. The DR for monosomy X is over 90% and false positive rate is 1%.
18323334 In a study using prospectively collected samples, PPV for monosomy X was 48.4% (30%–67%).
18 It should be conveyed that there can be a false positive result for sex chromosome abnormality, which may have varied clinical implication in the presence of maternal sex chromosome mosaicism.
35 The cfDNA screening for microdeletions is not recommended because the PPV ranges from 3.8 to 17%, which precludes practical implication.
36
Recommendation: 1) It should be clarified that a ‘low-risk’ result does not mean the absence of chromosomal abnormality. 2) A mother with a result of ‘no call’ should be offered the invasive test, rather than a retest (Level 2++, Grade B).
Summary of evidence: Although it shows good performance in the screening of trisomy 21, 18, and 13, several studies have reported false positive and false negative cases.
3738 Therefore, confirmative diagnosis should be made only after the invasive test has been performed. Likewise, women with a negative result should be informed that it does not rule out all abnormalities in fetal chromosomes.
39 In cases of a failure to obtain result (‘no call’), the woman is more likely to have fetal aneuploidy such as trisomy 18 and 13.
2840 The success rate of the repeat test is reported to be as low as 45%–50% or as high as 80%–90%, depending on the study setting.
41 Considering the higher risk of abnormal result and the anxiety of women who have to wait for the test to be repeated, we do not recommend repeating cfDNA screening in women with ‘no call’ result. This ‘no call’ result is more common in women with obesity, with the prevalence being 20% when the bodyweight is > 113 kg (250 lb) and 50% when it is > 159 kg (350 lb).
42 Thus, cfDNA screening would not be the optimal fetal aneuploidy screening test in pregnancy for women with severe obesity.
43
Recommendation: The cfDNA screening cannot be offered as a first-line aneuploidy screening test for all women because it is not cost-effective in low-risk pregnancy (Level 2++, Grade B).
Summary of evidence: On an average, cfDNA screening shows better performance in detecting chromosomal trisomy compared to the conventional maternal serum screening test.
40 However, PPV of cfDNA screening is lower in the low-risk group compared to the high-risk group. There is also the possibility of ‘no call,’ when the amount of placenta-derived cell-free DNA in maternal blood is not sufficient for the test. Comparing to the analyte-based tests, which are frequently positive for a large range of chromosomal abnormalities, cfDNA screening is specific for individual aneuploidies.
44 Of abnormal result from invasive diagnostic testing, 16.9% of all abnormalities detected by invasive diagnostic testing were considered undetectable by the current cfDNA screening.
45 Given the high cost of cfDNA screening, it is not cost-effective to recommend this test for all pregnant women.
Recommendation: The optimal timing for cfDNA screening is 10 weeks of gestation and beyond (Level 2++, Grade A).
Summary of evidence: The proportion of fetal component in cfDNA in maternal blood has been known to remain constant between gestational weeks 10 and 22, regardless of maternal ethnicity or age.
46 Recently, some reports have shown that the proportion of fetal component is closely associated with gestational age; fetal component in cfDNA increases by 0.1% per week during gestational weeks 10–21 and by 1% after gestational week 21.
4748 For most pregnant women, the proportion of fetal component is > 4% after gestational week 10, which is sufficient for cfDNA screening. The test is likely to fail to yield a result before gestational week 9, with only 72.6% success rate.
28 Therefore, it is recommended that cfDNA screening be performed after gestational week 10.
Recommendation: Cell-free DNA screening is not recommended for women with multiple gestations, since the accuracy in multiple pregnancy is not as high as that in singleton pregnancy (Level 1+, Grade B).
Summary of evidence: Twin pregnancy generally shows lower fetal component in cfDNA of maternal blood than singleton pregnancy (7.4% vs. 10% or 8.7% vs. 11.7%).
4950 The failure rate was also shown to be higher in twin pregnancy.
5051 Therefore, cfDNA screening is not recommended in twin pregnancy because of poorer performance compared to a singleton pregnancy.
Recommendation: Women with a high-risk result should be offered the option of having an invasive test. It should be urged that no irreversible obstetric procedures be undertaken without a confirmative diagnostic test (Level 2++, Grade B).
Summary of evidence: Given the false positive and false negative cases and the varied accuracy, depending on the type of aneuploidy, cfDNA screening should not be regarded as a confirmative test.
37394052 According to a study of 31,030 women, the PPV for all types of trisomy was 82.9% and for trisomy 21 it was 90.9%.
53 Therefore, drawing conclusions of fetal aneuploidy, based on a high-risk result without any invasive testing, should be avoided.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download