Abstract
This study investigated the impact of social distancing due to coronavirus disease 2019 (COVID-19) on glycemic control in people with type 2 diabetes mellitus (T2DM). We retrospectively analyzed the change in glycosylated hemoglobin level (ΔHbA1c) in people with T2DM who undertook social distancing because of COVID-19. We compared the ΔHbA1c between COVID-19 and non-COVID-19 cohorts that were enrolled at the same time of year. The ΔHbA1c of the COVID-19 cohort was significantly higher than that of two non-COVID-19 cohorts. Subgroup analysis according to age and baseline HbA1c level showed that social distancing significantly increased the mean HbA1c level of participants of <50 years. The ΔHbA1c of participants of <50 years and with HbA1c <7.0% in the COVID-19 cohort showed larger changes than other subgroups. In adjusted model, adjusted ΔHbA1c levels in the COVID-19 cohort remained significantly higher than those in the two other cohorts. Social distancing negatively impacts blood glucose control in people with T2DM, especially those who are younger and have good blood glucose control.
The spread of coronavirus disease 2019 (COVID-19) worldwide has resulted in changes in lifestyle in areas affected by COVID-19 [1,2]. To stem the transmission of COVID-19 infection, strategies aimed at reducing the frequency and closeness of contact between people are essential [3,4]. Following the detection of a super-spreader on February 18, 2020, the number of patients with COVID-19 rapidly increased in Daegu, which became the epicenter of the outbreak in South Korea [5]. As a consequence, the citizens of Daegu conducted a voluntary lock-down that lasted for approximately 2 months, and involved strict social distancing [6]. It is well established that lifestyle interventions are fundamental management tools for patients with diabetes [7,8], and it might be expected that social distancing would influence the blood glucose control of people with type 2 diabetes mellitus (T2DM). However, there have been no studies to date that have assessed the influence of social distancing on blood glucose control in people with T2DM. Therefore, we sought to determine the effects of social distancing because of COVID-19 on the changes in glycosylated hemoglobin (HbA1c) level in people with T2DM.
We conducted a retrospective cohort study of people with T2DM who attended one of five tertiary hospitals in Daegu during the following periods of time. The five hospitals included in this study are as follows; Kyungpook National University Hospital (KNUH) and Chilgok Hospital (KNUCH), Daegu Catholic University Hospital (DCUH), Yeungnam University Hospital (YUH), and Keimyung University Dongsan Hospital (KUDH). The flow chart of the study design is presented as Supplementary Fig. 1. The study period was divided up according to the timing of the COVID-19 outbreak in Daegu (February 18, 2020). Period 1 (November 18 to February 17) was defined as the period from 3 months before the outbreak until 1 day prior to the date of the outbreak, and Period 2 (February 18 to May 17) was defined as the 3 months following the start of the outbreak. Data were also collected for the same dates during the 2 previous years. Therefore, data were collected not only from the COVID-19 cohort (2019 to 2020), but also from the non-COVID-19 cohort 1 (2018 to 2019) and cohort 2 (2017 to 2018), and categorized into Periods 1 and 2. Of 74,636 T2DM patients who visited the hospitals and had their HbA1c levels measured during the designated periods, the patients in each cohort who had this parameter measured during both Periods 1 and 2 were enrolled. After excluding patients who were aged <19 years, a total of 20,087 patients were enrolled in the present study (5,069 at KNUH, 1,836 at KNUCH, 4,396 at DCUH, 3,064 at YUH, and 5,722 at KUDH). The age, sex, and HbA1c values for each patient were collected from their electronic medical records. The changes in glycosylated hemoglobin level (ΔHbA1c) between Periods 1 and 2 in the COVID-19 cohort were compared with those in the non-COVID-19 cohorts as control groups. The study was approved by the Institutional Review Boards of KNUH (2020-05-063-001) and individually by the Institutional Review Boards of each collaborating hospital. The necessity for informed consent was waived by the ethics boards of the hospitals because of the retrospective study design.
Statistical analysis was performed using SPSS version 18.0 software (SPSS Inc., Chicago, IL, USA). Analysis of variance was used to evaluate the differences in ΔHbA1c among the cohorts. Within each cohort, the difference in mean HbA1c level between Periods 1 and 2 was compared using a paired t-test. An adjusted ΔHbA1c value was estimated after adjustment for multiple confounding factors (age, sex, and baseline HbA1c) using analysis of covariance. P<0.05 was considered to indicate statistical significance.
The average age and age range for all participants and each cohort are as follows; total (62.8 years [19 to 95 years]), COVID-19 cohort (62.6 years [19 to 95 years]), non-COVID-19 cohort 1 (62.9 years [19 to 94 years]), and non-COVID-19 cohort 2 (62.9 years [19 to 93 years]). The characteristics of participants were presented in Table 1. The mean HbA1c levels of the non-COVID-19 cohorts were decreased in Period 2 than in Period 1 (P< 0.01), whereas the mean HbA1c level of the COVID-19 cohort did not differ between the two periods (P=0.26) (Table 1). The mean ΔHbA1c of the COVID-19 cohort was significantly higher than those of the non-COVID-19 cohorts (P<0.01) (Fig. 1).
Subgroup analysis was conducted to analyze the mean HbA1c according to sex, age, and baseline HbA1c (Table 1 and Fig. 1). The mean ΔHbA1c was higher in the COVID-19 cohort in both male and female participants. In subgroup analysis conducted according to age, the mean HbA1c level in participants aged <50 years significantly increased after social distancing commenced (P=0.04). Interestingly, ΔHbA1c differed most markedly between cohorts in participants of <50 years (P<0.01), whereas ΔHbA1c did not differ in participants of ≥70 years (P=0.23). In subgroup according to baseline HbA1c level, the difference in ΔHbA1c was more apparent in participants with low baseline HbA1c levels (P<0.01) and there was no significant difference for participants with an HbA1c of ≥9.0% at baseline (P=0.70).
After adjustment for age, sex, and baseline HbA1c level, the adjusted ΔHbA1c level in the COVID-19 cohort remained significantly higher than those in the two other cohorts (Supplementary Table 1). Subgroup analysis showed that difference in adjusted ΔHbA1c between COVID-19 cohort and two non-COVID-19 cohorts was more marked in participants of <50 years (P<0.01) and in participants with low baseline HbA1c levels (P<0.01).
In the present study, we have shown that the patterns of change in HbA1c level significantly differed between a COVID-19 cohort and two non-COVID-19 cohorts. After the start of the COVID-19 pandemic, strict social distancing was implemented to prevent the transmission of COVID-19 infection in South Korea [5]. Because Daegu city accounted for approximately 70% of the COVID-19 cases in South Korea, the social distancing in this region was more strict than in other regions of Korea [6]. Although the changes in lifestyle connected with social distancing may have a significant impact on individuals with diabetes, no studies to date have assessed the influence of social distancing on blood glucose control in people with T2DM. Contrary to a recent study reporting the improvement of blood glucose control in people with type 1 diabetes mellitus during the COVID-19 pandemic [9], our study showed that social distancing because of COVID-19 negatively impacts blood glucose control in people with T2DM. Thus, because the absence of a vaccine or effective medication for COVID-19 has created a requirement for prolonged social distancing, it is necessary to institute appropriate additional strategies for the control of blood glucose in people with T2DM.
We found that the mean HbA1c level in non-COVID-19 cohorts was significantly lower during Period 2 than Period 1. Several previous studies have shown that HbA1c is higher in colder seasons than in warmer seasons [10,11] and that this difference can be affected by physical activity and food intake [12]. The 18th of February, which was the date the outbreak commenced, represents the transition from winter to spring in South Korea; therefore, as shown in previous studies, a decrease might have been due to seasonal variation. However, the HbA1c level tended to increase in the COVID-19 cohort, which suggests that the seasonal variation in HbA1c level is affected by social distancing. We found that the increases in HbA1c level in subgroups of participants with an HbA1c ≤7.0% or who were <50 years of age were more marked during the period of social distancing than in the other subgroups. These data suggest that the deleterious effects of social distancing are more marked in the socially active T2DM patients. One strategy of glycemic control during periods of social distancing will be to frequently implement self-monitoring of blood glucose, especially in these individuals.
The present study had some limitations. First, only HbA1c level was analyzed to assess blood glucose control in people with T2DM. Second, several important clinical parameters affecting HbA1c level were not assessed in the present study (e.g., body mass index, duration of diabetes, comorbidities, and medications). Therefore, these confounding factors may influence the results of our study. Third, the short observation period was insufficient to fully reflect the changes in HbA1c level caused by social distancing. Finally, the results of the study cannot identify which particular aspects of social distancing may have effects on blood glucose control.
Despite these limitations, the study has generated meaningful findings. We suggest that social distancing due to COVID-19 negatively impacts glycemic control in people with T2DM. The deleterious effects of social distancing may be more pronounced in the socially active patients. Therefore, it is important that management strategies are modified for people with T2DM during periods of social distancing that are designed to minimize COVID-19 infection.
SUPPLEMENTARY MATERIALS
Supplementary materials related to this article can be found online at https://doi.org/10.4093/dmj.2020.0226.
Supplementary Table 1.
Estimated mean of change of glycosylated hemoglobin after adjustment for multiple confounding factors
Supplementary Fig. 1.
The flow chart of the study design. COVID-19, coronavirus disease 2019.
Notes
AUTHOR CONTRIBUTIONS
Conception or design: S.D.P., S.W.K., K.G.P.
Acquisition, analysis, or interpretation of data: S.D.P., S.W.K., J.S.M., Y.Y.L., N.H.C., J.H.L., J.H.J., Y.K.C., M.K.K., K.G.P.
Drafting the work or revising: S.D.P., S.W.K., J.S.M., Y.Y.L., N.H.C., J.H.L., J.H.J., Y.K.C., M.K.K., K.G.P.
Final approval of the manuscript: K.G.P.
REFERENCES
1. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. China Novel Coronavirus Investigating and Research Team. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020; 382:727–33.

2. Eurosurveillance editorial team. Updated rapid risk assessment from ECDC on the novel coronavirus disease 2019 (COVID-19) pandemic: increased transmission in the EU/EEA and the UK. Euro Surveill. 2020; 25:2003121.
3. Delen D, Eryarsoy E, Davazdahemami B. No place like home: cross-national data analysis of the efficacy of social distancing during the COVID-19 pandemic. JMIR Public Health Surveill. 2020; 6:e19862.

4. Badr HS, Du H, Marshall M, Dong E, Squire MM, Gardner LM. Association between mobility patterns and COVID-19 transmission in the USA: a mathematical modelling study. Lancet Infect Dis. 2020; 20:1247–54.



5. Korean Society of Infectious Diseases; Korean Society of Pediatric Infectious Diseases; Korean Society of Epidemiology; Korean Society for Antimicrobial Therapy; Korean Society for Healthcare-associated Infection Control and Prevention; Korea Centers for Disease Control and Prevention. Report on the epidemiological features of coronavirus disease 2019 (COVID-19) outbreak in the Republic of Korea from January 19 to March 2, 2020. J Korean Med Sci. 2020; 35:e112.
6. Park SW, Sun K, Viboud C, Grenfell BT, Dushoff J. Potential roles of social distancing in mitigating the spread of coronavirus disease 2019 (COVID-19) in South Korea. medRxiv. 2020. Mar. 30.
https://doi.org/10.1101/2020.03.27.20045815
.

7. Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, et al. Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001; 344:1343–50.

8. Boule NG, Haddad E, Kenny GP, Wells GA, Sigal RJ. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta-analysis of controlled clinical trials. JAMA. 2001; 286:1218–27.

9. Bonora BM, Boscari F, Avogaro A, Bruttomesso D, Fadini GP. Glycaemic control among people with type 1 diabetes during lockdown for the SARS-CoV-2 outbreak in Italy. Diabetes Ther. 2020; 11:1–11.


10. Liang WW. Seasonal changes in preprandial glucose, A1C, and blood pressure in diabetic patients. Diabetes Care. 2007; 30:2501–2.
11. Sakamoto M, Matsutani D, Minato S, Tsujimoto Y, Kayama Y, Takeda N, et al. Seasonal variations in the achievement of guideline targets for HbA(1c), blood pressure, and cholesterol among patients with type 2 diabetes: a nationwide population-based study (ABC Study: JDDM49). Diabetes Care. 2019; 42:816–23.


Fig. 1
The changes in glycosylated hemoglobin level (ΔHbA1c) of people with type 2 diabetes mellitus in the coronavirus disease 2019 (COVID-19) cohort and non-COVID-19 cohorts. ΔHbA1c was presented as mean±standard error. Data were analyzed using analysis of variance. NS, not significant, aP<0.01, bP<0.05.
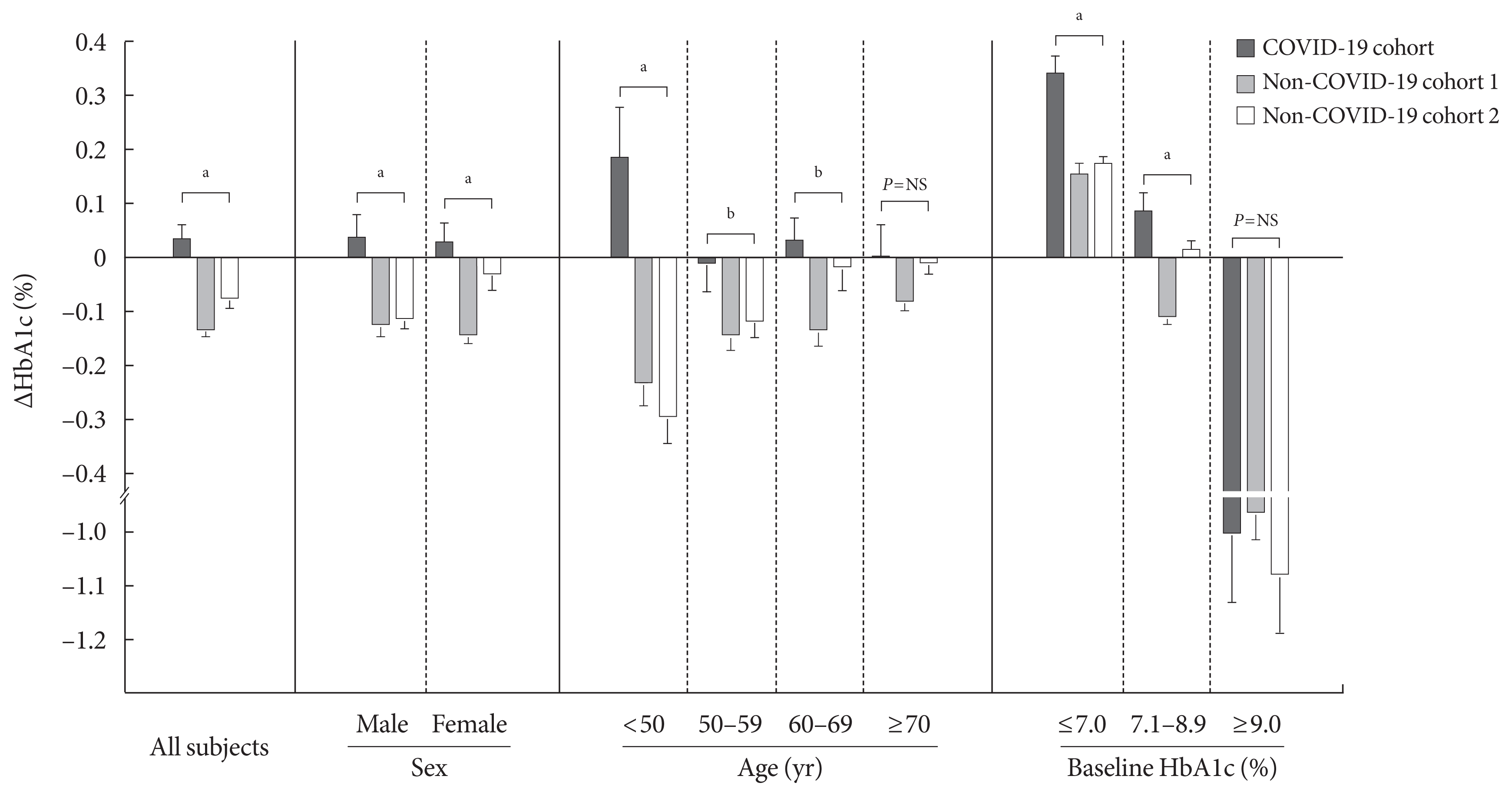
Table 1
Characteristics and glycated hemoglobin of people with type 2 diabetes mellitus in COVID-19 cohort and non-COVID-19 cohorts




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download