Abstract
This report presents the status of diabetic neuropathy (DN) in Korea as determined using a National Health Insurance Service-National Sample Cohort (NHIS-NSC). Annual prevalences of DN were estimated by age and gender using descriptive statistics. Pharmacological treatments for DN were also analyzed. The annual prevalence of DN increased from 24.9% in 2006 to 26.6% in 2007, and thereafter, gradually subsided to 20.8% in 2015. In most cases, pharmacological treatments involved a single drug, which accounted for 91.6% of total prescriptions in 2015. The most commonly used drugs (in decreasing order) were thioctic acid, an anti-convulsive agent, or a tricyclic antidepressant. In conclusion, the prevalence of DN decreased over the 10-year study period. Thioctic acid monotherapy was usually prescribed for DN. To reduce the socio-economic burden of DN, more attention should be paid to the diagnosis of this condition and to the appropriate management of patients.
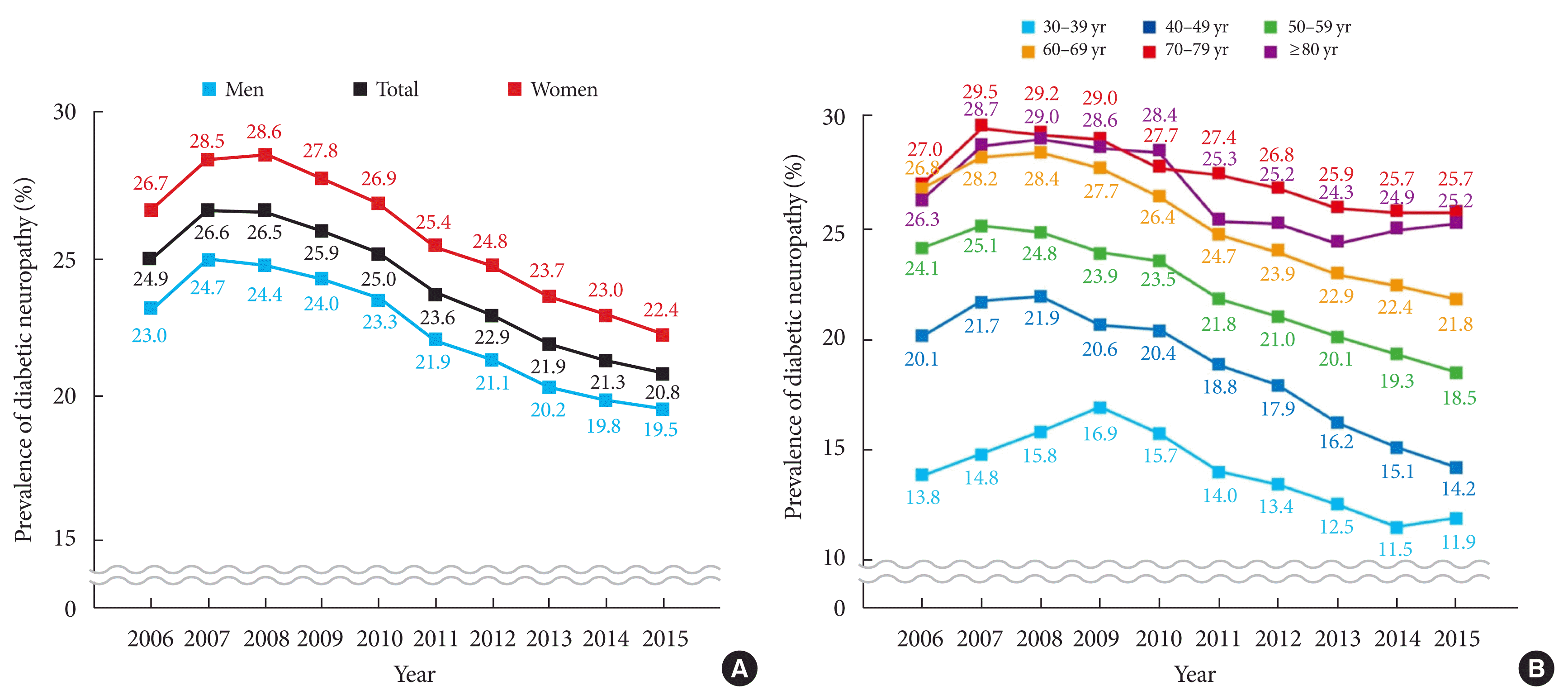
• The prevalence of diabetic neuropathy over a decade(2006-2015) increased initially and then decreased later from 26.6% to 20.8%.
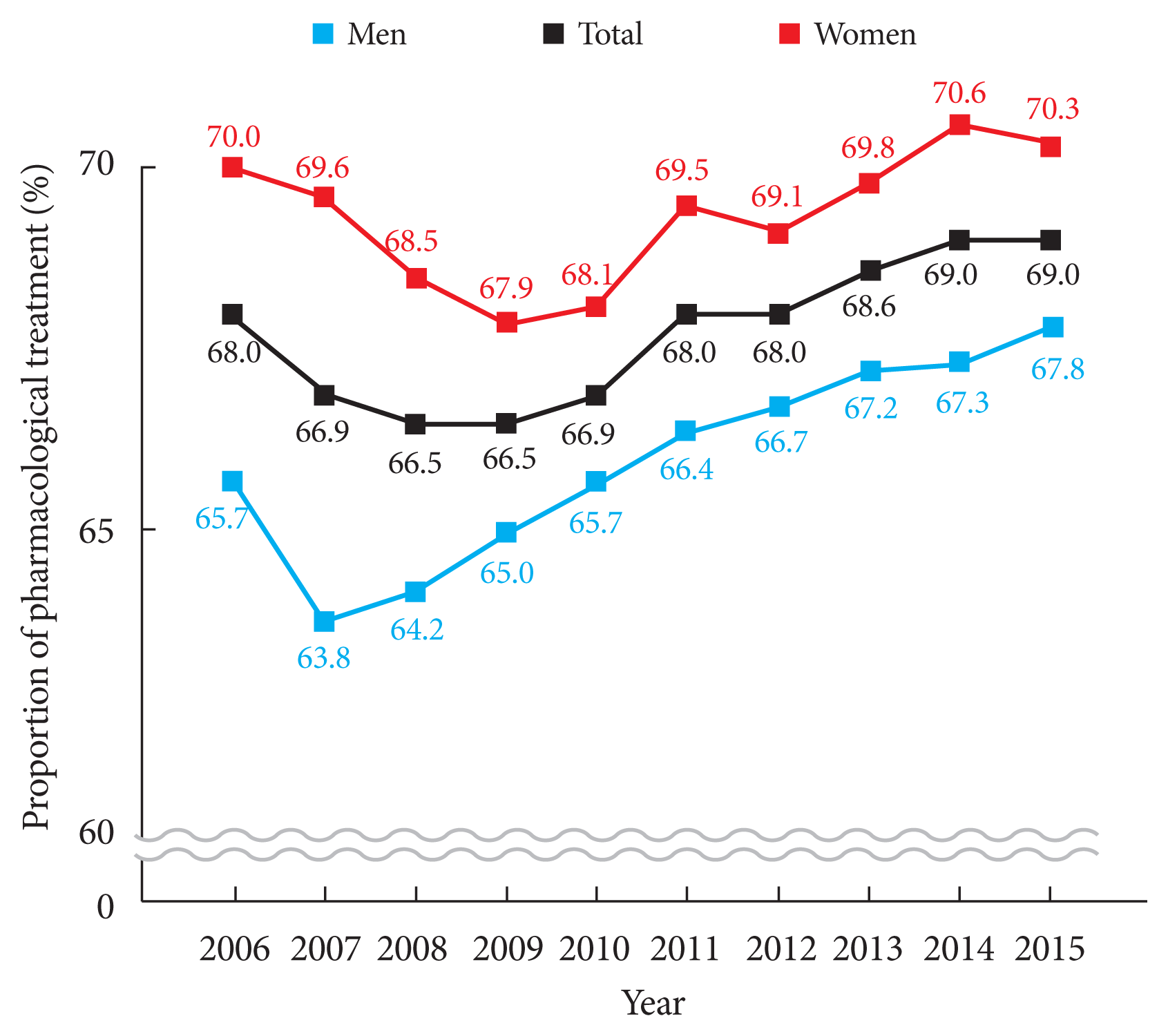
• The rate of pharmacologic treatment for diabetic neuropathy decreased early in the decade and then gradually increased since then, with the rate of drug treatment ranging from 66.5% to 69.0%.
• About 91% of all prescriptions were monotherapies and combination therapies constituted <10%.
• Thioctic acid was the most commonly prescribed drug type followed in order by anti-convulsive agents and tricyclic antidepressants.
The management of diabetes mellitus has evolved and several newly developed anti-diabetic drugs are now available that disrupt its underlying mechanisms [1,2]. However, the majority of the patients fail to achieve treatment targets sufficiently enough to prevent diabetic complications [3,4]. Diabetic neuropathy (DN), which are one of the most common chronic complication of diabetes [5], can involve a spectrum of nerve fibers including peripheral sensory nerves, cranial nerves, and autonomic nerves [6]. Among them, diabetic peripheral neuropathy is the most prevalent form of DN [7] and can impair quality of life by causing pain, mental disorders, and functional abnormalities [8] and lead to devastating complications and the socioeconomic burden [9,10]. Previous epidemiologic studies have reported prevalences of diabetic peripheral neuropathy ranging from 23% to 50% [11–14]. Recently, the prevalence of diabetic peripheral neuropathy in the Korean population was reported to be 33.5% in a nationwide hospital-based multicenter study conducted by the Diabetic Neuropathy Study Group of the Korean Diabetes Association [15]. However, it should be noted that the design of this study limited its ability to represent the real status of DN in the Korean diabetic population. In this study, we investigated DN prevalences, patient characteristics, and pharmacological treatments using a National Health Insurance Service-National Sample Cohort (NHIS-NSC).
The Korean NHIS is a compulsory single-payer national health care insurance system that covers 98% of the population residing within the territory of Korea and manages all health service utilization databases [16,17]. The NHIS-NSC was instituted in 2006 and involves random sampling of 2.2% of the total eligible population, and followed until 2015 [18]. For this study, we selected individuals aged ≥30 years with diabetes from January 1, 2006 to December 31, 2015 from the NHIS-NSC. Diabetes was defined as a diagnosis of diabetes (as defined by the 10th edition of the International Classification of Diseases [ICD-10] codes E10−E14) on more than two occasions, receipt of a prescription for oral glucose-lowering drugs (Anatomical Therapeutic Chemical [ATC] code A10B) for >30 days, or receipt of an insulin (ATC code A10A) prescription on an outpatient basis in each year (Supplementary Table 1). The study protocol was reviewed and approved by the Institutional Review Board of Ajou University Hospital (IRB No. AJIRB-MED-EXP-18-333), which waived the requirement for informed consent because all patient data were de-identified.
We defined the presence of DN as follows: (1) a diagnosis of DN (ICD-10 codes E10.4, E11.4, E12.4, E13.4, E14.4, G59.0, G63.2, and G99.0), or (2) receipt of a prescription for glucose-lowering drugs (ATC code A10B or A10A) plus drugs for DN (ATC codes A16AX01, D11AX02, N06AA10, N06AA09, N03AX12, N03AX16, and N06AX21) (Supplementary Table 1). Crude prevalence rates were calculated as percentages among individuals with diabetes. Age-adjusted prevalence rates were standardized against the year 2011 weighted distribution of study people with diabetes across sexes and 5-year age groups from 30 to 34 to 85 years and over.
Treatment for DN was defined as the receipt of a prescription of any medication for DN at least once per year. The medication possession ratio (MPR) that reflects adherence was defined as the case of person who receives medication for DN prescriptions for at least 290 days (80%) per year. In cases where the medication prescribed was changed several times, the prescription with the longest duration in a given year was taken to be representative. The analysis was conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
The prevalence of DN was 24.9% among adult (≥30-year-old) patients with diabetes in 2006, peaked at 26.6% in 2007, and then consistently and gradually declined to 20.8% in 2015. Women had a higher prevalence than men by 3% to 4% throughout the study period (Fig. 1A). Prevalence tended to increase with age, though it was greater in those aged 70 to 79 years than in those aged ≥80 years. The prevalences of DN in those aged 70 to 79 years and 30 to 39 years were 25.7% and 11.9%, respectively (Fig. 1B).
Over the 10-year study period, the pharmacological prescription rate ranged between 66.5% and 69.0% (Fig. 2). Monotherapy accounted for 91.6% of all prescriptions in 2015 (Supplementary Fig. 1). Of the several classes of drugs prescribed, thioctic acid was administered most. Notably, the prescription of anti-convulsive agents consistently increased during the study period, whereas the prescriptions of thioctic acid and tricyclic antidepressants gradually decreased (Supplementary Fig. 2). In 2015, dual combination therapy accounted for 8.1% of total prescriptions. (Supplementary Fig. 3). MPR for DN consistently increased from 30.0% in 2006 to 49.0% in 2015 (Supplementary Fig. 4).
The prevalence of DN ranged from 20.8% to 26.6% during 10-year study period which is lower than the 33.5% found in a recent multicenter study conducted by the DN study group of the Korean Diabetes Association [15]. Such discrepancies are probably due to different study populations and the lack of standard diagnostic criteria. In the present study, DN was defined using diagnostic codes or medications prescribed by clinicians rather than using clinical diagnostic criteria and neurologic examinations. Therefore, the prevalences of DN determined in the present study may have been underestimated.
Interestingly, we found the prevalence of DN showed a decreasing trend, whereas the prevalences of other microvascular diseases such as diabetic retinopathy (men, from 12.6 to 15.1; women, from 14.7 to 17.4 per 100 adults with diabetes) and diabetic nephropathy (men, from 8.6 to 12.9; women, from 8.1 to 11.8 per 100 adults with diabetes) have shown increasing trends based on NHIS-NSC database analysis over the same study period [18]. The reason for this is unclear because pathogenetic mechanisms of microvascular complications of diabetes are common and have common risk factors [19]. However, unlike retinopathy and nephropathy, the standard diagnostic methodology for DN has not been well established and its course is insidious, and thus, DN has been underdiagnosed as compared with other diabetic complications. However, a decreasing trend was reported for proliferative diabetic retinopathy (men, from 138 to 126; women, from 120 to 104 per 10,000 adults with diabetes) [19], and in the same cohort, the prevalence rates of major cardiovascular complications, including ischemic heart disease and stroke, tended to decline [19], which is similar to that observed in the present study from 2006 to 2015. The prevalence of DN was 24.9% among adult patients with diabetes in 2006, peaked at 26.6% in 2007, and then consistently and gradually declined until 2015. It is unclear why the prevalence showed that ‘up and down’ pattern while we could guess that it might not be due to an increased prevalence of DN but because of an increased diagnosis with more attention to DN. Follow-up of NHIS-NSC data over a greater period is required to confirm this decreasing trend in the prevalence of DN.
The prescription rate for DN over the study period ranged from 66.5% to 69.0%. About 91% of all prescriptions were monotherapies and combination therapies constituted <10%. We suspect that a lack of NHIS coverage for combination drug therapy until 2015 partly explains this disparity. Thioctic acid was the most commonly prescribed drug type followed in order by anti-convulsive agents and tricyclic antidepressants. This prescription pattern is unique because most academic groups recommend anti-convulsive agents and selective serotonin-norepinephrine reuptake inhibitors as 1st or 2nd line therapies for diabetic peripheral neuropathy [20].
The present study has a number of limitations that warrant consideration. Our definition of DN is not perfect so that we could not exclude the possibility of over or underestimate the prevalence of DN. Although we included ICD-10 codes of diabetic autonomic neuropathy in the definition of DN, the diagnosed cases might be rare in real-world considering the complexity of diagnosis of diabetic autonomic neuropathy. We could not include all drugs for DN. Considering the innate limitation of the NHIS data and real status in diabetic clinics, we included diagnosis codes related to DN and most commonly used drugs for the definition to avoid under and overestimate the prevalence, respectively. Time-associated changes in age distribution of NHIS-NSC possibly contributed to the observed reduction in the prevalence of DN during the study period. Nevertheless, this is the first study to present annual changes in the prevalence of DN in Korea in a nationally representative cohort in the diabetic population. To prevent devastating consequences and reduce the socioeconomic burden of DN further studies are warranted to determine more precisely the national status of DN.
SUPPLEMENTARY MATERIALS
Supplementary materials related to this article can be found online at https://doi.org/10.4093/dmj.2020.0120.
Supplementary Table 1
Anatomical therapeutic chemical code for drugs of diabetic neuropathy
Supplementary Fig. 1
Trends of pharmacologic treatment for diabetic neuropathy.
Supplementary Fig. 2
Trends of monotherapy drugs for diabetic neuropathy. SNRI, serotonin-norepinephrine reuptake inhibitor;
TCA, tricyclic antidepressant.
Supplementary Fig. 3
The trend of dual combination therapy in diabetic neuropathy. SNRI, serotonin-norepinephrine reuptake
inhibitor; TCA, tricyclic antidepressant.
Supplementary Fig. 4
Medication possession rate, continuity of pharmacologic treatment for diabetic neuropathy.
Notes
AUTHOR CONTRIBUTIONS
Conception or design: S.S.M., C.H.K., T.S.P.
Acquisition, analysis, or interpretation of data: S.S.M., C.H.K., S.M.K., E.S.K., T.J.O., J.S.Y., H.C.C., D.J.K., T.S.P.
Drafting the work or revising: S.S.M., C.H.K., D.J.K., T.S.P.
Final approval of the manuscript: S.S.M., C.H.K., S.M.K., E.S.K., T.J.O., J.S.Y., H.C.C., D.J.K., T.S.P.
ACKNOWLEDGMENTS
This study used NHIS data (NHIS-2020-2-051) made by National Health Insurance Service (NHIS). The authors declare no conflict of interest with NHIS. We fully acknowledge the participating members of the Korean Diabetic Neuropathy Study Group.
REFERENCES
1. American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2019. Diabetes Care. 2019; 42(Suppl 1):S90–102.
2. American Diabetes Association. 7. Diabetes technology: standards of medical care in diabetes-2019. Diabetes Care. 2019; 42(Suppl 1):S71–80.
3. Won JC, Lee JH, Kim JH, Kang ES, Won KC, Kim DJ, et al. Diabetes fact sheet in Korea, 2016: an appraisal of current status. Diabetes Metab J. 2018; 42:415–24.



4. Ko SH, Han K, Lee YH, Noh J, Park CY, Kim DJ, et al. Past and current status of adult type 2 diabetes mellitus management in Korea: a National Health Insurance Service database analysis. Diabetes Metab J. 2018; 42:93–100.



5. Dyck PJ, Kratz KM, Karnes JL, Litchy WJ, Klein R, Pach JM, et al. The prevalence by staged severity of various types of diabetic neuropathy, retinopathy, and nephropathy in a population-based cohort: the Rochester Diabetic Neuropathy Study. Neurology. 1993; 43:817–24.


6. Sasaki H, Kawamura N, Dyck PJ, Dyck PJB, Kihara M, Low PA. Spectrum of diabetic neuropathies. Diabetol Int. 2020; 11:87–96.



7. Javed S, Hayat T, Menon L, Alam U, Malik RA. Diabetic peripheral neuropathy in people with type 2 diabetes: too little too late. Diabet Med. 2020; 37:573–9.

8. Kim SS, Won JC, Kwon HS, Kim CH, Lee JH, Park TS, et al. Prevalence and clinical implications of painful diabetic peripheral neuropathy in type 2 diabetes: results from a nationwide hospital-based study of diabetic neuropathy in Korea. Diabetes Res Clin Pract. 2014; 103:522–9.


9. Vinik AI, Nevoret ML, Casellini C, Parson H. Diabetic neuro pathy. Endocrinol Metab Clin North Am. 2013; 42:747–87.

10. U. K. Prospective Diabetes Study Group. Quality of life in type 2 diabetic patients is affected by complications but not by intensive policies to improve blood glucose or blood pressure control (UKPDS 37). Diabetes Care. 1999; 22:1125–36.

11. Candrilli SD, Davis KL, Kan HJ, Lucero MA, Rousculp MD. Prevalence and the associated burden of illness of symptoms of diabetic peripheral neuropathy and diabetic retinopathy. J Diabetes Complications. 2007; 21:306–14.


12. Gregg EW, Sorlie P, Paulose-Ram R, Gu Q, Eberhardt MS, Wolz M, et al. Prevalence of lower-extremity disease in the US adult population >=40 years of age with and without diabetes: 1999–2000 national health and nutrition examination survey. Diabetes Care. 2004; 27:1591–7.

13. Adler AI, Boyko EJ, Ahroni JH, Stensel V, Forsberg RC, Smith DG. Risk factors for diabetic peripheral sensory neuropathy. Results of the Seattle Prospective Diabetic Foot Study. Diabetes Care. 1997; 20:1162–7.


14. Cabezas-Cerrato J. The prevalence of clinical diabetic polyneuropathy in Spain: a study in primary care and hospital clinic groups. Neuropathy Spanish Study Group of the Spanish Diabetes Society (SDS). Diabetologia. 1998; 41:1263–9.

15. Won JC, Kwon HS, Kim CH, Lee JH, Park TS, Ko KS, et al. Prevalence and clinical characteristics of diabetic peripheral neuropathy in hospital patients with type 2 diabetes in Korea. Diabet Med. 2012; 29:e290–6.
16. Song SO, Jung CH, Song YD, Park CY, Kwon HS, Cha BS, et al. Background and data configuration process of a nationwide population-based study using the Korean national health insurance system. Diabetes Metab J. 2014; 38:395–403.



17. Lee YH, Han K, Ko SH, Ko KS, Lee KU. Taskforce Team of Diabetes Fact Sheet of the Korean Diabetes Association. Data analytic process of a nationwide population-based study using National Health Information Database established by National Health Insurance Service. Diabetes Metab J. 2016; 40:79–82.



18. Korean Diabetes Association. Diabetes fact sheet in Korea. Available from: https://www.diabetes.or.kr/pro/news/admin.php?mode=list&category=B(cited 2020 Oct 16).
19. Barrett EJ, Liu Z, Khamaisi M, King GL, Klein R, Klein BEK, et al. Diabetic microvascular disease: an Endocrine Society scientific statement. J Clin Endocrinol Metab. 2017; 102:4343–410.



20. American Diabetes Association. 11. Microvascular complications and foot care: standards of medical care in diabetes-2019. Diabetes Care. 2019; 42(Suppl 1):S124–38.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download