Abstract
Background
There is little longitudinal information on psychological burden and metabolic outcomes in young adults with diabetes (YAD) in Asia. We aimed to evaluate the association between psychological status and glycemia at baseline and 2 years following transition in a cohort of YAD in Singapore.
Methods
Subjects with type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM), aged 17 to 25 years, were recruited from the YAD clinic in Singapore General Hospital. The Hospital Anxiety and Depression and Problem Areas for Diabetes scales were administered at transition (baseline) and at 18 to 24 months. Glycosylated hemoglobin (HbA1c) assessed during routine visits was tracked longitudinally.
Results
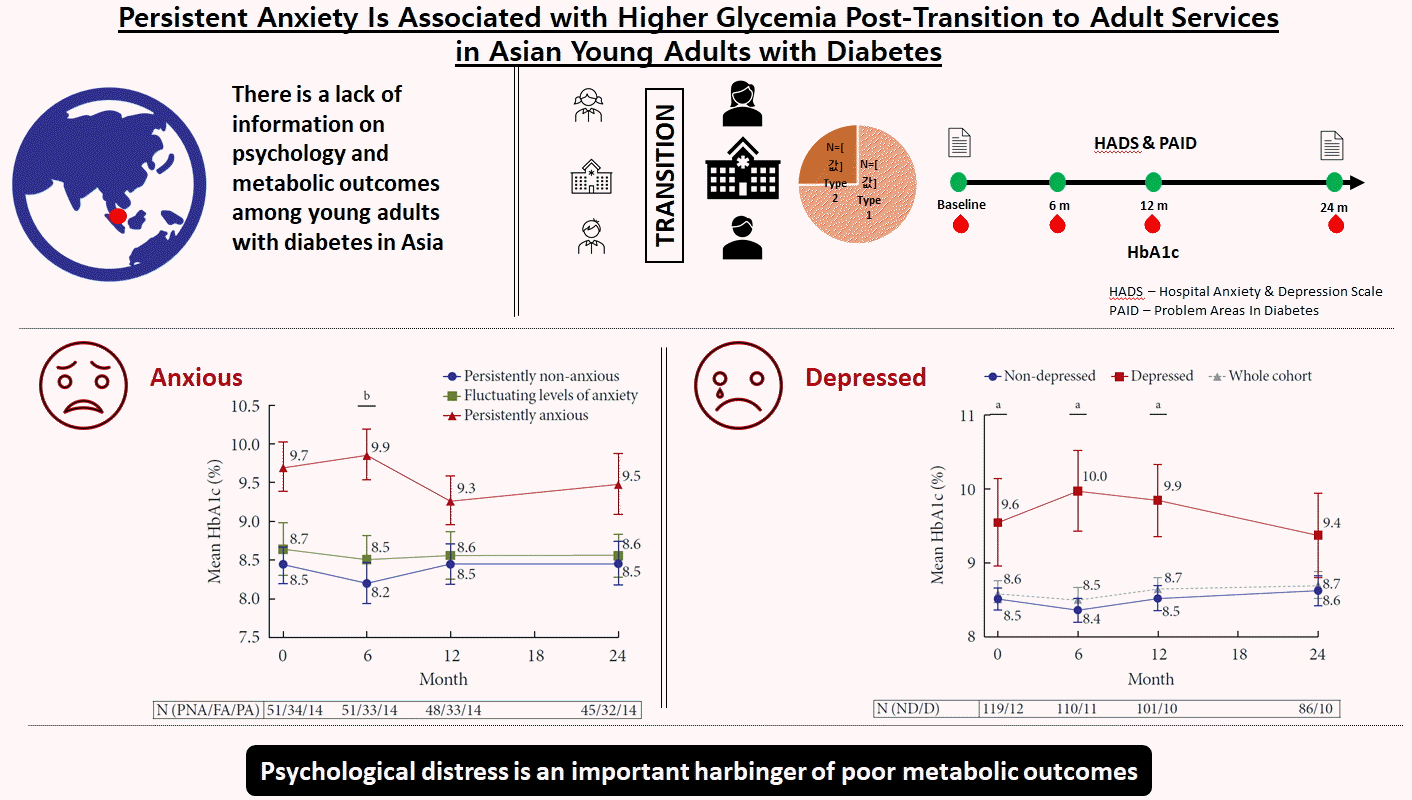
A total of 98 T1DM (74.8%) and 33 T2DM (25.2%) subjects were recruited between January 2011 and November 2017. At baseline, mean HbA1c was 8.6%±1.7%. Only 26.0% achieved HbA1c of ≤7.5% and 16.8% achieved HbA1c of <7%. At baseline, prevalence of anxiety was 29.8%. At 24 months, 14.1% had persistent anxiety. Those with persistent anxiety had the highest mean HbA1c, particularly at 6 months (persistently anxious vs. persistently non-anxious: 9.9%±1.2% vs. 8.2%±1.9%, P=0.009). At baseline, 9.2% of subjects had depression. This group also had poorer glycemia at baseline (HbA1c of depressed vs non-depressed: 9.6%±2.1% vs. 8.5%±1.6%, P=0.04), which persisted up to 24 months.
The prevalence of diabetes increased worldwide in the last four decades, and particularly so in Asia, where the largest and fastest increase has been observed [1]. More worryingly, this diabetes epidemic is marked by a rapidly expanding population of patients with young onset diabetes [2]. At least 18% of individuals with type 2 diabetes mellitus (T2DM) in the Joint Asia Diabetes Evaluation (JADE) Program were diagnosed below the age of 40 years [3]. The younger age at diabetes onset means a longer exposure to hyperglycemia and potential for accrual of diabetes-related complications. The trend toward increasing prevalence and disease burden of diabetes in young adults (aged 18 to 30 years) emphasizes the need to optimize diabetes care in this group [2].
The transition from pediatric-oriented to adult care contributes to challenges in managing diabetes in young adults [4]. Transition goes beyond a simple shift in geographical location and healthcare providers. It involves natural physiological changes that occur in this age group and psychosocial changes from transformations in the paradigm of care, both from the parents' and the young adults' perspectives [56]. This period also coincides with competing academic, economic, and social priorities. The rapidly evolving societal identities and roles of these individuals may induce vulnerability and may lower the prioritization of diabetes self-care [4].
Unsurprisingly, young adulthood is often characterized by deteriorating glycemia. Data from the Type 1 Diabetes Exchange registry demonstrated that the peak mean glycosylated hemoglobin (HbA1c) of 9.2% occurs at age 19 years [7]. Similarly, data from Europe and Oceania showed that young adults with type 1 diabetes mellitus (T1DM) were less likely to achieve the HbA1c target of <7% and <7.5% as recommended by the International Society for Pediatric and Adolescent Diabetes and American Diabetes Association, respectively [891011]. In Asia, the literature on metabolic outcomes of young adults with diabetes (YAD), particularly those with T1DM, is scarce. The JADE Program demonstrated that patients with young onset of T2DM had longer disease duration, higher mean HbA1c, and higher rates of diabetes complications than those with older onset of diabetes (after age 40 years) [3].
The deterioration in metabolic parameters during young adulthood highlights the presence of factors beyond those managed through conventional treatment, a critical component of which is mental health. Rates of depression (10% to 35%) and anxiety (9% to 21%) in adolescents with diabetes are reportedly high [121314]. To date, most psychological studies have been cross-sectional or have a limited follow-up. Accordingly, there have been few longitudinal evaluations to quantify the long-term effects of psychological burden on metabolic outcomes. This is particularly so in Asia, where the mental health of this vulnerable age group is not well-studied, and structured transition from pediatric to adult care is often not well-established [15].
In this study, we aimed to characterize the trajectory of the glycemia of YAD following entry into the Young Adult with Diabetes clinic in Singapore General Hospital, Singapore. We also aimed to assess the prevalence of anxiety and depression in this cohort at baseline and at 24 months following transition, and its relationship with glycemia. We hypothesized that anxiety and depression would associate with poorer glycemia and a higher rate of diabetes-related hospitalizations.
Ethical committee approval for this study was obtained from the SingHealth Centralised Institutional Review Board in 2011 (CIRB 2011/503/C).
Subjects aged 17 to 25 years with T1DM and T2DM attending the YAD clinic were recruited between January 2011 and November 2017. Pregnant women, prisoners, and subjects above the age of 25 years at entry into the clinic were excluded. The data collected forms part of an ongoing longitudinal observational study of diabetes self-management, psychological status, and metabolic parameters among YAD in Singapore. All eligible subjects had the opportunity to clarify queries regarding the study before providing consent. Parental consent was also obtained for subjects under 21 years of age.
The YAD clinic at Singapore General Hospital was set up in January 2011; its initial purpose was to provide a structured transition service for pediatric patients with diabetes who needed to move toward adult-oriented care. The clinic since expanded to accept all 17- to 25-year-old with diabetes.
As part of routine clinical care, all first-visit subjects had a review of their diabetes diagnosis and management to date, knowledge of diabetes self-management, and need for financial or counseling aid. Diabetes subtype allocations were reviewed. Insulin users with positive antibodies to glutamic acid decarboxylase (anti-GAD Ab), and/or a random C-peptide level of <0.2 nmol/L were classified as having T1DM [161718]. Subjects who did not fulfill these criteria were managed as T2DM.
Follow-up visits took place every 3 to 4 months. The clinic provided a one-stop geographical set-up in which the endocrinologist, diabetes nurse educator, dietitian, and medical social worker tended to the subjects based on their needs. Individuals with T1DM were encouraged to attend the Singapore Dose Adjustment for Normal Eating (SgDAFNE) Programme, a structured education program for T1DM patients on self-management and advanced carbohydrate counting [19]. As part of the routine clinical care, subjects who expressed major depressive symptoms or distress were referred to the psychiatrist for mental health evaluation and treatment.
Data collection was between January 2011 and February 2018. Demographics and clinical characteristics of subjects were obtained from hospital electronic health records at the point of entry into the YAD clinic including sex, ethnicity, height, weight, and body mass index (BMI), diabetes subtype, and the presence of albuminuria and retinopathy. Digital retinal photography was performed annually. Images were graded and reported by a centralized reading center. Screening for albuminuria also occurred annually. The presence of albuminuria was defined as a microalbumin:creatinine ratio on a spot urine of >3.4 mg/mmol on more than one occasion [20], or treatment with an angiotensin converting enzyme inhibitor or angiotensin receptor antagonist for the condition anytime during the study period.
HbA1c was measured using a National Glycohemoglobin Standardization Program (NGSP)-certified immunoassay (Tina-quant Gen 3; Roche Diagnostics, Basel, Switzerland) [21]. These HbA1c values were retrieved at clinic entry, 6, 12, and 24 months after transition. Diabetes-related hospital admissions during the study period were collated from hospital medical records. Clinic attendance rates were computed by dividing the attended visits by the total number of scheduled visits multiplied by 100.
Psychological status of recruited subjects was measured using the Hospital Anxiety and Depression Scale (HADS) at transition and 18 to 24 months into follow-up. HADS is a 14-item questionnaire with two subscales, Anxiety (HADS-A) and Depression (HADS-D). Subscale scores were computed from the sum of the respective subscales' item responses. A score of 0 to 7 is regarded as normal, 8 to 10 as mild, and 11 to 21 as moderate to severe levels of the respective states [22]. In this study, anxiety and depression were defined as HADS-A ≥8 and HADS-D ≥8, respectively. Diabetes-related distress was assessed using the Problem Areas in Diabetes Scale (PAID). A total score of ≥40 was used as the threshold to indicate significant diabetes-related distress [23].
All analyses were performed using IBM SPSS Statistics version 21.0 for Windows (IBM Co., Armonk, NY, USA). Comparisons between diabetes subtypes, HbA1c, and anxiety or depression groups were performed using a chi-square test or independent samples two-tailed Student's t-test where appropriate. A P≤0.05 was considered statistically significant. Graphs were generated using GraphPad Prism version 8.2.1 for Windows (GraphPad Software, San Diego, CA, USA).
A total of 155 YAD attending the YAD clinic were screened between January 2011 and November 2017, and 131 subjects were recruited (Supplementary Fig. 1). The cohort comprised 69.5% Chinese, 13.7% Indian, and 14.5% Malay ethnicities (Table 1), and 98 (74.8%) subjects had T1DM. Within this cohort of young adults (mean age of 19.6±2.1 years) with diabetes (mean duration of diabetes 8.2±5.0 years), a substantial proportion had albuminuria (14.5%) and diabetic retinopathy (6.1%). At baseline, mean HbA1c of the T1DM group and T2DM group were 8.5%±1.3% and 9.0%±2.3%, respectively. Pre-transition HbA1c (up to 6 months) was available in 127 of 131 subjects. In T1DM subjects, HbA1c was higher pre-transition compared with transition baseline (8.9%±2.1% vs. 8.5%±1.3%, P=0.002) but unchanged in T2DM (9.2%±2.6% vs. 9.0%±2.3%, P=0.68). Despite a significantly shorter duration of diabetes (4.9±4.0 years vs. 9.3±4.8 years, P <0.001), a greater proportion of the T2DM group had poor glycemia at baseline (proportion with HbA1c >10%, T2DM vs. T1DM: 30.3% vs. 11.2%, P=0.02). The T2DM group also had a higher mean BMI (27.6±5.9 kg/m2 vs. 22.4±3.0 kg/m2, P <0.001).
Prevalence of anxiety and depression in the cohort of 131 subjects was assessed at baseline. A comparison of demographic and clinical characteristics between groups with and without anxiety and depression is presented in Table 2. Anxiety was present in 39 (29.8%) of the subjects. Sixteen (12.2%) of the cohort demonstrated moderate to severe anxiety (HADS-A ≥11). The mean HADS-A scores in the T1DM and T2DM groups were 6.2±3.6 and 5.5±3.9 (P=0.32), respectively. Depression was less prevalent (12 [9.2%]) than anxiety. Four (3.1%) displayed moderate to severe depression (HADS-D ≥11). The mean HADS-D scores in T1DM and T2DM groups were 3.1±3.1 and 3.3±3.0 (P=0.75), respectively. Ten (7.6%) subjects had both anxiety and depression.
The mean PAID score in this cohort was 28.6±19.2, with 38 (29.0%) of the subjects scoring ≥40. Subjects from the anxious (A) group had significantly higher PAID scores than subjects from non-anxious (NA) group (40.0±17.9 vs. 23.7±17.7, P<0.001). Similarly, the depressed (D) group had higher PAID scores than the non-depressed (ND) group (43.2±22.9 vs. 27.1±18.3, P<0.005). Correlation analysis revealed moderate correlation between PAID and HbA1c at both transition (r=0.3, P<0.01) and 24 months (r=0.35, P<0.01).
Ninety-nine subjects had a psychological assessment at both transition and 24-month follow-up. There were no significant differences in HADS-A and HADS-D scores between T1DM and T2DM at 24 months. Subjects were grouped according to change in anxiety status at 24 months. Those whose HADS-A scores were <8 at both transition and 24 months were categorized as persistently non-anxious (PNA). Subjects with HADS-A scores ≥8 at both transition and 24 months were categorized as persistently anxious (PA). Subjects whose anxiety status changed over 24 months, either from anxious to non-anxious or vice versa, were deemed to have fluctuating levels of anxiety (FA). Although the majority (51.5%) of the cohort remained free from symptoms of anxiety at 24 months, 34 (34.3%) had FA, with more subjects moving from NA to A (20 [20.2%]) than from A to NA (14 [14.1%]). Fourteen (14.1%) had PA from transition to 24 months into follow-up.
Similarly, subjects were grouped according to change in depression status at 24 months. Although the majority (81.8%) of the cohort remained free from symptoms of depression at 24 months, more subjects moved from ND to D (8 [8.1%]), than from D to ND (5 [5.1%]). Five (5.1%) had persistent depression. These results suggest a trend toward worsening anxiety and depression scores following transition in this cohort.
At baseline, there was no difference in glycemia between subjects with anxiety and subjects without anxiety (A vs. NA, HbA1c: 8.9%±1.7% vs. 8.5%±1.6%, P=0.28) (Fig. 1A). This between-group difference in HbA1c increased at 6 months and reached statistical significance (A vs. NA, HbA1c: 9.1%±1.8% vs. 8.3%±1.7%, P=0.025). Thereafter, the difference decreased and was no longer significant from 12 months onward. The correlations between HADS-A scores and HbA1c were weak at both transition and 24 months and not statistically significant.
We compared the HbA1c between the three anxiety change groups (PA, PNA, and FA), and demonstrated more apparent differences in glycemia. Subjects who were PA had considerably higher HbA1c than subjects in the PNA and FA groups. This difference was the greatest at 6 months (PA vs. PNA, HbA1c: 9.9%±1.2% vs. 8.2%±1.9%, P=0.009). This difference in HbA1c levels between the PA and PNA groups was observed to persist through 24 months, although this was not statistically significant (Fig. 1B). No significant changes in HbA1c were noted between the group that moved from NA to A, and the group that moved from A to NA.
In addition to worse glycemia, the PA group was also characterized by significantly higher PAID scores at baseline and 24-month follow-up (47.4±15.5 and 45.6±20.8, respectively), compared with the FA (32.6±17.2 and 32.4±21.0, respectively) and PNA groups (21.1±16.3 and 18.1±13.0, respectively).
Subjects with depression at transition had poorer glycemia at baseline compared with subjects without depression (D vs. ND, HbA1c: 9.6%±2.1% vs. 8.5%±1.6%, P=0.04) (Fig. 2). This finding remained statistically significant throughout the first 12 months, with the difference in HbA1c between the D and ND groups being the greatest at 6 months (D vs. ND, HbA1c: 10.0%±1.8% vs. 8.4%±1.7%, P=0.004). We did not find any strong or significant correlation between HADS-D scores and HbA1c at transition or 24 months.
We analyzed the differences in HbA1c between subjects grouped according to change in depression status at 24 months. There were no significant differences in glycemia between the PD, PND and FD groups at any time point (Supplementary Fig. 2).
Hospitalization rates for hyperglycemic crises and severe hypoglycemia from the PNA, FA, and PA groups were not significantly different (6 [11.8%], 5 [14.7%], and 2 [14.3%], respectively). A higher hospitalization rate was noted in the D group (4 [40.0%]), compared with the ND group (9 [10.1%], P <0.05). Clinic attendance census per annum increased from 138 visits in 2011 to 437 visits in 2017, with a no-show rate ranging from 17.1% to 25.5% (average no-show rate of 21.0% over 7 years). There was no significant difference in outpatient clinic attendance rates between the PNA, FA, and PA groups (84.9%±19.6%, 84.0%±18.4%, and 80.2%±21.7%, respectively; P= 0.73). Similarly, no statistically significant difference was found in clinic attendance rates between the ND and D groups (84.4%±19.7% vs. 79.1%±18.6%, P=0.5).
This prospective observational study in YAD, managed within a multidisciplinary team in Singapore, is the first to report on glycemia and prevalence of diabetes-related complications at baseline within this age group in Southeast Asia. We demonstrated that at transition, glycemia was suboptimal in the majority of the cohort. We also demonstrated the association among anxiety, depression and glycemia of YAD at transition and the trajectory of these relationships 24 months later.
At both baseline and 24 months later, approximately one-third (29.8%) of this group of YAD had anxiety, and 14.1% remained PA. Notably, anxiety was observed to correlate with a higher HbA1c level at baseline, with subjects who remained PA consistently having higher HbA1c throughout the 24 months of observation. These rates of anxiety in the current cohort are higher than the reported 9% to 21% from the United States and Europe [132425]. This could be related to the much lower incidence of T1DM in Asian countries (e.g., incidence of T1DM between the ages of 0 and 14 years: 27.9 per 100,000 in Norway, 2.5 per 100,000 in Singapore) [226]. Given the low incidence and hence lower awareness of T1DM in Asia, the psychological burden of an individual living with T1DM in Asia would perceivably be substantial [27]. Furthermore, among the T1DM and insulin-treated T2DM groups, the complexity of tools required in diabetes management may lead to stigma [2829]. It has been shown that stigma is prevalent in YAD and is associated with poor metabolic outcomes [30].
The relationship between anxiety and diabetes self-management in young adults is complex. First, it is difficult to ascertain if symptoms of anxiety in YAD are from living with the chronic condition or from other competing interests in late adolescence. Analysis of the prevalence of anxiety in young adults with and without diabetes may provide insights into the potential role of chronic disease in the development of anxiety. However, such data is scarce, and there is heterogeneity in the instruments employed to assess anxiety symptoms in subjects with diabetes [31]. A Norwegian population-based study found no significant differences in mental health scores between adolescents with T1DM and adolescents without [32]. These results may not be immediately applicable to Asia because of the lower prevalence of T1DM in Asia and the different healthcare systems and cultural contexts [226]. Few studies have investigated whether diabetes could lead to incident anxiety, and others have proposed that the relationship between diabetes and anxiety is likely bidirectional [33].
Over the 2-year follow-up period, we have demonstrated that anxiety at baseline is associated with poorer glycemia. Subjects with PA consistently fared worse than the NA group and the group with FA. There was an early divergence in HbA1c between subjects with and without anxiety, particularly at 6-month post-transition. This finding could be a reflection of the physical and psychological challenges during the transition period. With attendance in the multidisciplinary adult services post-transition, some of the gaps in knowledge and skillsets required for diabetes self-management could be addressed. This may explain the fall in HbA1c in the PNA group and the difference in glycemic control between the PA versus PNA group at 6 months. Through the 24 months there remains a trend toward higher HbA1c levels in subjects who were PA versus subjects who were PNA. The non-significance of the difference in HbA1c at the end of 24-month follow-up might be related to the sample size. This data seems to suggest that regardless of the direction of causality, it would be important to systematically screen for and address anxiety promptly because of the association of persistent anxiety with poorer glycemia.
The prevalence of depression was 9.2% in this cohort of young adults, which is lower than the 10% to 35% reported from North America and Australia [121314]. This difference could be related to the use of different evaluation methods to detect depression (e.g., the Patient Health Questionnaire [PHQ-8/9]). We used the HADS-D, which is well-validated internationally and has been found to be a robust screening tool for mental disorders [34]. PHQ-9 may also overestimate depression in subjects with diabetes because of the inclusion of items such as symptoms of diabetes [35]. Second, there are cultural differences in expression of depressive symptoms, with the Asian culture encouraging resilience and withstanding of hardship, making the diagnosis of depression more challenging [3637].
Subjects with symptoms of depression had significantly higher HbA1c in the first 12 months following transition. This observation echoes the finding that metabolic outcome is worse in T1DM and T2DM individuals with depression and predicts worse glycemia [38]. The mechanism and direction of the associations between the two pathologies are not well understood. There could be a reciprocal relationship [39]. Interventional studies on YAD are limited; hence, the effectiveness of treatment of depression in improving glycemia in this age group should be demonstrated in further research.
Notably, clinic attendance rates were comparable across all anxiety and depression status groups, suggesting that the difference in glycemia between these groups was unlikely caused by a lack of diabetes self-care education or access to healthcare. As clinic attendance alone does not directly translate into behavioral change, additional strategies and interventions targeted at each patient's unique needs are crucial. For instance, a psychology-based approach designed to support the youths to better cope with their chronic condition might further complement care provided by the hospital multidisciplinary team. Sustainability of outcomes of interventions is also a critical consideration in the planning and provision of psychosocial support [40].
Our study is the first of its type to be conducted in Asia. Using the dataset from one of the largest cohorts of YAD in Singapore, we reported comprehensively on the glycemic outcomes, clinic attendance rate, hospitalization rate, diabetes complication rate, and psychological scores of 131 subjects. Through a structured multidisciplinary care team, we worked closely with our subjects to achieve mean attendance rates of over 80%. This clinic attendance rate is comparable to the reported rate of up to 75% by other transition centers [41]. The ability to track both HbA1c and anxiety and depression scores longitudinally allowed a delineation of change of psychological status and glycemia. Specifically, we demonstrated an early divergence in HbA1c between those with and without anxiety, particularly at 6-month post-transition. Prompt identification, efforts to retain patients, and directing of resources in the immediate post-transition period may be important in ameliorating anxiety in YAD and would hopefully improve outcomes.
Our study has limitations. We have focused on HbA1c as the main measure of glycemia. Capturing other parameters including glycemic variability and incidence of hypoglycemia would have provided a more comprehensive assessment of glycemia. Glycemia can also be influenced by treatment modality. Although the majority of our cohort was treated with multiple dose insulin injection therapy during the study, we did not control for treatment modality in our analyses. Since the inception of our study, there has been a change in landscape of diabetes technology. Thus, further research could consider the influence of diabetes treatment modalities on glycemia. Although we have demonstrated the association between persistent anxiety and poor glycemia, it remains unclear if a psychological intervention targeting persistent anxiety or depression improves glycemic outcomes. We have grouped subjects according to their HADS-A and HADS-D scores but acknowledge that there is great heterogeneity even within these categories. Some individuals with high HADS scores may have better coping mechanisms than others within the same anxiety or depression category. A myriad of biopsychosocial factors has been demonstrated to impact glycemia. We acknowledge that it might have been useful to expand the collection of baseline demographic data of our cohort to include information such as socioeconomic status and education level [4243]. Although dietetics assessment forms an important element of routine care in our YAD clinic, we did not systematically collate and quantify dietary, alcohol, and smoking history in our study. Anxiety and depression can also coexist with other psychological co-morbidities, such as eating disorders [44]. It has been demonstrated that eating disorders are associated with poorer glycemia [45]. Qualitative interviews in these subgroups would also further the understanding of the interactions between psychological welfare and outcomes.
In conclusion, among this group of young Singaporeans with diabetes, one-third had anxiety or depressive symptoms at transition. There is an early increase of HbA1c at 6 months, followed by persistent poor glycemia in this group with baseline anxiety or depression, despite equal access to a multidisciplinary care team. An effective psychology-based intervention is necessary to improve the outcomes of this group of YAD during transition.
Notes
ACKNOWLEDGMENTS
We would like to thank the staff of the Diabetes and Metabolism Centre, Singapore General Hospital, for their support and contributions, as well as patients of the Young Adult Diabetes clinic for their participation in this study.
References
1. NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016; 387:1513–1530.
2. International Diabetes Federation. IDF diabetes atlas. 8th ed. Brussels: International Diabetes Federation;2017.
3. Yeung RO, Zhang Y, Luk A, Yang W, Sobrepena L, Yoon KH, et al. Metabolic profiles and treatment gaps in young-onset type 2 diabetes in Asia (the JADE programme): a cross-sectional study of a prospective cohort. Lancet Diabetes Endocrinol. 2014; 2:935–943.


4. Peters A, Laffel L. American Diabetes Association Transitions Working Group. Diabetes care for emerging adults: recommendations for transition from pediatric to adult diabetes care systems. A position statement of the American Diabetes Association, with representation by the American College of Osteopathic Family Physicians, the American Academy of Pediatrics, the American Association of Clinical Endocrinologists, the American Osteopathic Association, the Centers for Disease Control and Prevention, Children with Diabetes, The Endocrine Society, the International Society for Pediatric and Adolescent Diabetes, Juvenile Diabetes Research Foundation International, the National Diabetes Education Program, and the Pediatric Endocrine Society (formerly Lawson Wilkins Pediatric Endocrine Society). Diabetes Care. 2011; 34:2477–2485.


5. Daneman D, Nakhla M. Moving on: transition of teens with type 1 diabetes to adult care. Diabetes Spectr. 2011; 24:14–18.

6. Adams JD, Hayes J, Hopson B. Transition: understanding and managing personal change. London: Martin Robertson;1976.
7. Miller KM, Foster NC, Beck RW, Bergenstal RM, DuBose SN, DiMeglio LA, et al. T1D Exchange Clinic Network. Current state of type 1 diabetes treatment in the US: updated data from the T1D Exchange clinic registry. Diabetes Care. 2015; 38:971–978.

8. McKnight JA, Wild SH, Lamb MJ, Cooper MN, Jones TW, Davis EA, et al. Glycaemic control of type 1 diabetes in clinical practice early in the 21st century: an international comparison. Diabet Med. 2015; 32:1036–1050.

9. Petitti DB, Klingensmith GJ, Bell RA, Andrews JS, Dabelea D, Imperatore G, et al. SEARCH for Diabetes in Youth Study Group. Glycemic control in youth with diabetes: the SEARCH for diabetes in Youth Study. J Pediatr. 2009; 155:668–672.



10. DiMeglio LA, Acerini CL, Codner E, Craig ME, Hofer SE, Pillay K, et al. ISPAD Clinical Practice Consensus Guidelines 2018: glycemic control targets and glucose monitoring for children, adolescents, and young adults with diabetes. Pediatr Diabetes. 2018; 19 Suppl 27:105–114.


11. American Diabetes Association. 13. Children and adolescents: standards of medical care in diabetes. 2019. Diabetes Care. 2019; 42 Suppl 1:S148–S164.
12. Lewis K, Hermayer K. All grown up: moving from pediatric to adult diabetes care. Am J Med Sci. 2013; 345:278–283.

13. Bernstein CM, Stockwell MS, Gallagher MP, Rosenthal SL, Soren K. Mental health issues in adolescents and young adults with type 1 diabetes: prevalence and impact on glycemic control. Clin Pediatr (Phila). 2013; 52:10–15.


14. Hislop AL, Fegan PG, Schlaeppi MJ, Duck M, Yeap BB. Prevalence and associations of psychological distress in young adults with type 1 diabetes. Diabet Med. 2008; 25:91–96.


15. Onda Y, Nishimura R, Morimoto A, Sano H, Utsunomiya K, Tajima N, et al. Diabetes Epidemiology Research International (DERI) Mortality Study Group. DERI) Mortality Study Group. Age at transition from pediatric to adult care has no relationship with mortality for childhood-onset type 1 diabetes in Japan: Diabetes Epidemiology Research International (DERI) Mortality Study. PLoS One. 2016; 11:e0150720.
16. Ng WY, Lee YS, Todd AL, Lui KF, Loke KY, Thai AC. Tyrosine phosphatase-like protein (IA-2) and glutamic acid decarboxylase (GAD65) autoantibodies: a study of Chinese patients with diabetes mellitus. Autoimmunity. 2002; 35:119–124.


17. Ko GT, Chan JC, Yeung VT, Chow CC, Li JK, Lau MS, et al. Antibodies to glutamic acid decarboxylase in young Chinese diabetic patients. Ann Clin Biochem. 1998; 35(Pt 6):761–767.


18. The DCCT Research Group. Effects of age, duration and treatment of insulin-dependent diabetes mellitus on residual beta-cell function: observations during eligibility testing for the Diabetes Control and Complications Trial (DCCT). J Clin Endocrinol Metab. 1987; 65:30–36.

19. DAFNE Study Group. Training in flexible, intensive insulin management to enable dietary freedom in people with type 1 diabetes: dose adjustment for normal eating (DAFNE) randomised controlled trial. BMJ. 2002; 325:746.
20. Tuttle KR, Bakris GL, Bilous RW, Chiang JL, de Boer IH, Goldstein-Fuchs J, et al. Diabetic kidney disease: a report from an ADA Consensus Conference. Diabetes Care. 2014; 37:2864–2883.



21. National Glycohemoglobin Standardization Program: Certified methods and laboratories. cited 2020 May 20. Available from: http://www.ngsp.org/certified.asp.
23. Welch GW, Jacobson AM, Polonsky WH. The problem areas in diabetes scale. An evaluation of its clinical utility. Diabetes Care. 1997; 20:760–766.


24. Li C, Barker L, Ford ES, Zhang X, Strine TW, Mokdad AH. Diabetes and anxiety in US adults: findings from the 2006 Behavioral Risk Factor Surveillance System. Diabet Med. 2008; 25:878–881.


25. Kampling H, Mittag O, Herpertz S, Baumeister H, Kulzer B, Petrak F. Can trajectories of glycemic control be predicted by depression, anxiety, or diabetes-related distress in a prospective cohort of adults with newly diagnosed type 1 diabetes? Results of a five-year follow-up from the German multicenter diabetes cohort study (GMDC-Study). Diabetes Res Clin Pract. 2018; 141:106–117.


26. Lee WR. The changing demography of diabetes mellitus in Singapore. Diabetes Res Clin Pract. 2000; 50 Suppl 2:S35–S39.

27. Jaacks LM, Liu W, Ji L, Mayer-Davis EJ. Type 1 diabetes stigma in China: a call to end the devaluation of individuals living with a manageable chronic disease. Diabetes Res Clin Pract. 2015; 107:306–307.


28. Browne JL, Ventura A, Mosely K, Speight J. ‘I’m not a druggie, I’m just a diabetic’: a qualitative study of stigma from the perspective of adults with type 1 diabetes. BMJ Open. 2014; 4:e005625.

29. Liu NF, Brown AS, Folias AE, Younge MF, Guzman SJ, Close KL, et al. Stigma in people with type 1 or type 2 diabetes. Clin Diabetes. 2017; 35:27–34.



30. Brazeau AS, Nakhla M, Wright M, Henderson M, Panagiotopoulos C, Pacaud D, et al. Stigma and its association with glycemic control and hypoglycemia in adolescents and young adults with type 1 diabetes: cross-sectional study. J Med Internet Res. 2018; 20:e151.

31. Rechenberg K, Whittemore R, Grey M. Anxiety in youth with type 1 diabetes. J Pediatr Nurs. 2017; 32:64–71.


32. Sivertsen B, Petrie KJ, Wilhelmsen-Langeland A, Hysing M. Mental health in adolescents with type 1 diabetes: results from a large population-based study. BMC Endocr Disord. 2014; 14:83.

33. Smith KJ, Deschenes SS, Schmitz N. Investigating the longitudinal association between diabetes and anxiety: a systematic review and meta-analysis. Diabet Med. 2018; 35:677–693.



34. Ali GC, Ryan G, De Silva MJ. Validated screening tools for common mental disorders in low and middle income countries: a systematic review. PLoS One. 2016; 11:e0156939.

35. Reddy P, Philpot B, Ford D, Dunbar JA. Identification of depression in diabetes: the efficacy of PHQ-9 and HADS-D. Br J Gen Pract. 2010; 60:e239–e245.

36. Zhang Y, Ting RZ, Yang W, Jia W, Li W, Ji L, et al. China Depression in Chinese Patients with Type 2 Diabetes (DD2) Study Group. Depression in Chinese patients with type 2 diabetes: associations with hyperglycemia, hypoglycemia, and poor treatment adherence. J Diabetes. 2015; 7:800–808.
38. Lustman PJ, Anderson RJ, Freedland KE, de Groot M, Carney RM, Clouse RE. Depression and poor glycemic control: a meta-analytic review of the literature. Diabetes Care. 2000; 23:934–942.


39. Mezuk B, Eaton WW, Albrecht S, Golden SH. Depression and type 2 diabetes over the lifespan: a meta-analysis. Diabetes Care. 2008; 31:2383–2390.



40. de Wit M, Delemarre-van de Waal HA, Bokma JA, Haasnoot K, Houdijk MC, Gemke RJ, et al. Follow-up results on monitoring and discussing health-related quality of life in adolescent diabetes care: benefits do not sustain in routine practice. Pediatr Diabetes. 2010; 11:175–181.


41. Fisher E, Lazar L, Shalitin S, Yackobovitch-Gavan M, de Vries L, Oron T, et al. Association between glycemic control and clinic attendance in emerging adults with type 1 diabetes: a tertiary center experience. J Diabetes Res. 2018; 2018:9572817.

42. Shulman R, Luo J, Shah BR. Mental health visits and low socio-economic status in adolescence are associated with complications of type 1 diabetes in early adulthood: a population-based cohort study. Diabet Med. 2018; 35:920–928.


43. Semenkovich K, Patel PP, Pollock AB, Beach KA, Nelson S, Masterson JJ, et al. Academic abilities and glycaemic control in children and young people with type 1 diabetes mellitus. Diabet Med. 2016; 33:668–673.


44. Young-Hyman D, de Groot M, Hill-Briggs F, Gonzalez JS, Hood K, Peyrot M. Psychosocial care for people with diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016; 39:2126–2140.


45. Nip ASY, Reboussin BA, Dabelea D, Bellatorre A, Mayer-Davis EJ, Kahkoska AR, et al. SEARCH for Diabetes in Youth Study Group. Disordered eating behaviors in youth and young adults with type 1 or type 2 diabetes receiving insulin therapy: the SEARCH for Diabetes in Youth Study. Diabetes Care. 2019; 42:859–866.



SUPPLEMENTARY MATERIALS
Supplementary materials related to this article can be found online at https://doi.org/10.4093/dmj.2019.0226.
Supplementary Fig. 1
Flow chart on recruitment and numbers analysed. HbA1c, glycosylated hemoglobin.
Supplementary Fig. 2
Mean glycosylated hemoglobin (HbA1c) over 24 months by pattern of depression status change. Error bar represents standard error of mean. No significant differences between groups were found.
Fig. 1
(A) Mean glycosylated hemoglobin (HbA1c) over 24 months by anxiety status at baseline. Mean HbA1c of the whole cohort is denoted by a gray dotted line. (B) Mean HbA1c over 24 months based on the pattern of anxiety status change. Error bar represents standard error of mean. aRepresents P <0.05 for comparisons between the non-anxious (NA) and anxious (A) groups. N (NA/A) denotes number of subjects in the NA and A groups, respectively, bRepresents P <0.05. N (PNA/FA/PA) denotes the number of subjects in the persistently non-anxious (PNA), fluctuating levels of anxiety (FA), and persistently anxious (PA) groups, respectively.
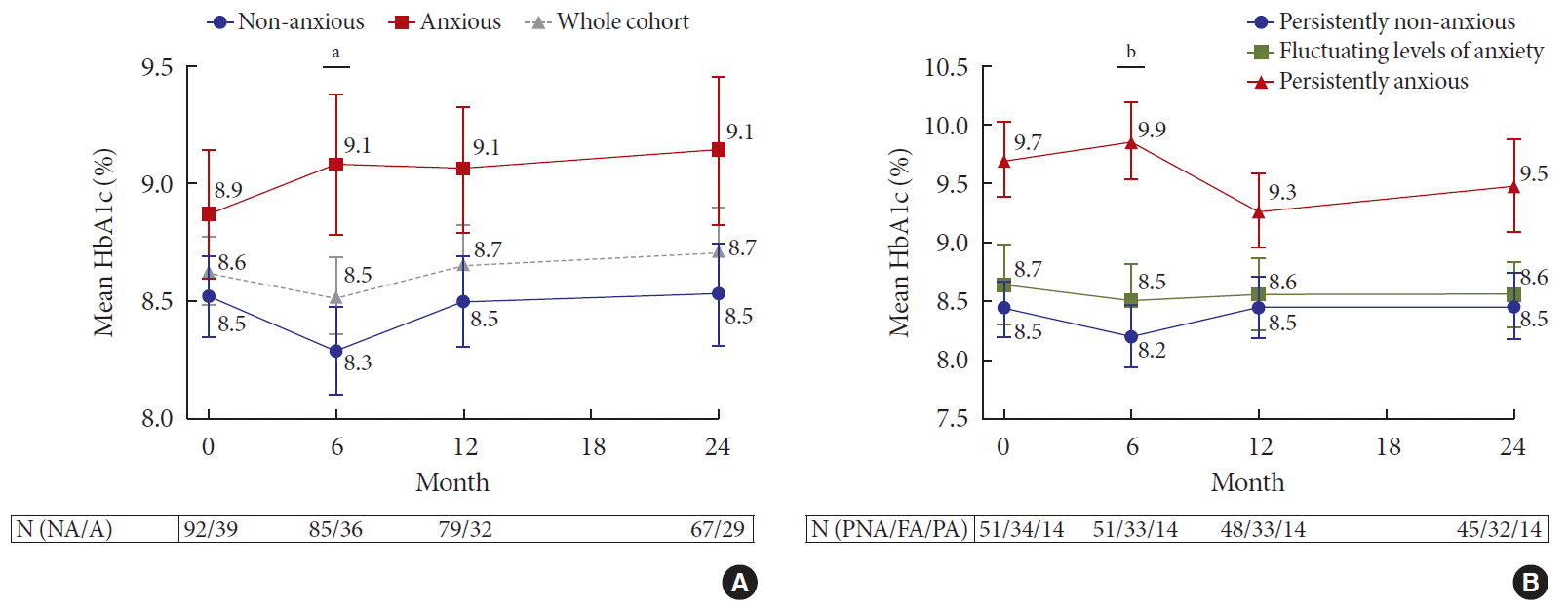
Fig. 2
Mean glycosylated hemoglobin (HbA1c) over 24 months by depression status at baseline. Mean HbA1c of the whole cohort is denoted by a gray dotted line. Error bar represents standard error of mean. aRepresents P <0.05 for comparisons between the non-depressed (ND) and depressed (D) groups. N (ND/D) denotes the number of subjects in the ND and D groups, respectively.
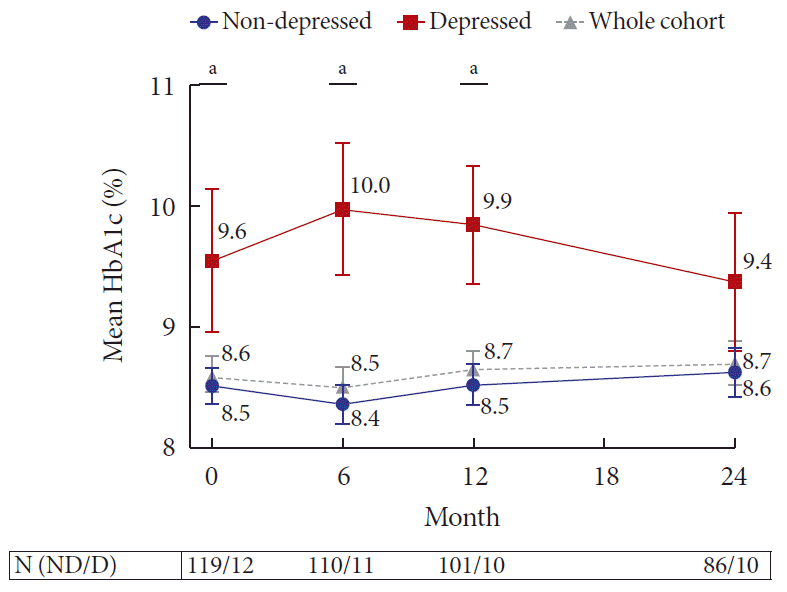
Table 1.
Demographics and baseline characteristics of the study cohort, and by diabetes subtype
| Characteristic | Study cohort (n=131) | T1DM (n=98, 74.8%) | T2DM (n=33, 25.2%) | P value |
|---|---|---|---|---|
| Age, yr | 19.6±2.1 | 19.6±2.0 | 19.7±2.6 | 0.74a |
| BMI, kg/m2 | 23.7±4.5 | 22.4±3.0 | 27.6±5.9 | <0.001a |
| Duration of disease, yr | 8.2±5.0 | 9.3±4.8 | 4.9±4.0 | <0.001a |
| Sex | 0.07b | |||
| Female | 74 (56.5) | 60 (61.2) | 14 (42.4) | |
| Male | 57 (43.5) | 38 (38.8) | 19 (57.6) | |
| Ethnicity | 0.72b | |||
| Chinese | 91 (69.5) | 66 (67.3) | 25 (75.8) | |
| Indian | 18 (13.7) | 15 (15.3) | 3 (9.1) | |
| Malay | 19 (14.5) | 15 (15.3) | 4 (12.1) | |
| Others | 3 (2.3) | 2 (2.1) | 1 (3.0) | |
| HbA1c, % | 8.6±1.7 | 8.5±1.3 | 9.0±2.3 | 0.12a |
| No. of subjects with HbA1c | 0.02b | |||
| <7.5% | 34 (26.0) | 25 (25.5) | 9 (27.3) | |
| >7.5% to ≤10% | 76 (58.0) | 62 (63.3) | 14 (42.4) | |
| >10% | 21 (16.0) | 11 (11.2) | 10 (30.3) | |
| Presence of albuminuria | 0.09b | |||
| Yes | 19 (14.5) | 11 (11.2) | 8 (24.2) | |
| No | 112 (85.5) | 87 (88.8) | 25 (75.8) | |
| Presence of retinopathy | 1.0b | |||
| Yes | 8 (6.1) | 6 (6.1) | 2 (6.1) | |
| No | 123 (93.9) | 92 (93.9) | 31 (93.9) |
Table 2.
Comparison of demographics and status of diabetes at transition, according to presence of anxiety and depression at baseline
| Variable | Anxious (n=39) | Non-anxious (n=92) | P value | Depressed (n=12) | Non-depressed (n=119) | P value |
|---|---|---|---|---|---|---|
| Age, yr | 19.7±2.2 | 19.6±2.1 | 0.66a | 20.2±2.1 | 19.6±2.1 | 0.35a |
| BMI, kg/m2 | 23.9±4.0 | 23.6±4.7 | 0.70a | 24.4±4.2 | 23.6±4.5 | 0.59a |
| Sex | 0.90b | |||||
| Female | 21 (53.8) | 53 (57.6) | 0.69b | 7 (58.3) | 67 (56.3) | |
| Male | 18 (46.2) | 39 (42.4) | 5 (41.7) | 52 (43.7) | ||
| Diabetes subtype | 0.99b | |||||
| T1DM | 30 (76.9) | 68 (73.9) | 0.72b | 9 (75.0) | 89 (74.8) | |
| T2DM | 9 (23.1) | 24 (26.1) | 3 (25.0) | 30 (25.2) | ||
| Duration of disease, yr | 7.9±4.7 | 8.3±5.2 | 0.66a | 8.6±5.0 | 8.1±5.0 | 0.76a |
| HbA1c, % | 8.9±1.7 | 8.5±1.6 | 0.28a | 9.6±2.1 | 8.5±1.6 | 0.04a |
| Presence of albuminuria | 0.01b | |||||
| Yes | 10 (25.6) | 9 (9.8) | 0.02b | 5 (41.7) | 14 (11.8) | |
| No | 29 (74.4) | 83 (90.2) | 7 (58.3) | 105 (88.2) | ||
| Presence of retinopathy | 0.74b | |||||
| Yes | 4 (10.3) | 4 (4.3) | 0.20b | 1 (8.3) | 7 (5.9) | |
| No | 35 (89.7) | 88 (95.7) | 11 (91.7) | 112 (94.1) |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download