Introduction
Procalcitonin (PCT) is a precursor of the hormone calcitonin. Being an acute-phase reactant, elevated PCT levels correlate strongly with inflammatory cytokines and the severity of bacterial infection [
1,
2]. The increase in PCT in response to viral and noninfectious inflammation is much less marked.
PCT levels rise early after an inflammatory challenge and peak at 6–14 hours [
1]. The half-life of PCT is 25–30 hours [
1]. Originally, PCT levels were measured for the diagnosis of bacterial vs. non-bacterial etiology of systemic inflammation. As experience with the marker increased, it has been used in a variety of clinical conditions [
1,
2]. The primary use has been for the confirmation, assessment of severity, and follow-up after initiation of therapy for sepsis, severe sepsis, and septic shock. PCT levels correlate well with organ dysfunction and the risk of mortality in patients admitted to critical care for sepsis. Another important use of PCT is to monitor the duration of antibiotic therapy in patients with sepsis. PCT has been used in obstetrics for detecting asymptomatic bacteriuria, infection associated with the premature rupture of membranes, and hypertensive diseases [
3–
6]. More recently, PCT has been used to detect pregnancy associated sepsis (PAS), as well as to differentiate between the severe and nonsevere cases [
7,
8].
The challenges of maternal morbidity and mortality from PAS continue to remain high in both, low-income and developed nations [
9]. The rate of PAS in developed countries ranges from to 4–10 per 10,000 live births. Although the exact data are not available, it is estimated that maternal mortality from PAS might be as high as 1% to 4.6% [
9].
Sepsis is usually associated with a cascade involving cytokine and toxin release, low perfusion, anoxia, and tissue inflammation and damage. Involvement of various organs and their dysfunction unfortunately remains the leading cause of mortality following PAS. Determination of the risk of organ dysfunction and assessment of the severity of PAS is necessary to initiate interventions and for prognostication. A biochemical investigation to aid clinical judgments would be an asset for the management of PAS. We assessed whether a repeat 48-hour PCT level in addition to its level at admission has a role in detecting organ dysfunction and mortality in patients with PAS.
Go to :

Materials and methods
A prospective observational study was conducted between January 2019 and December 2019 (12 months). Clearance from the Institutional Ethics Committee and prior consent was obtained from all patients. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
1. Inclusion criteria
All consecutive obstetric patients (pregnant, post-abortal [≥2 weeks] and postpartum [≤6 weeks]) with clinical signs of sepsis fulfilling the quick Sequential Organ Failure Assessment (qSOFA) criteria were enrolled as subjects with PAS.
2. Exclusion criteria
Subjects with a previously known history or diagnosed pathology of the pulmonary, cardiac, renal, hepatobiliary, and nervous system were excluded. Patients with asymptomatic bacteriuria, pre-eclampsia/eclampsia, premature rupture of the membranes, trauma, and burns were also excluded.
3. Methodology
For the purpose of this study, sepsis was defined as “life threatening organ dysfunction caused by a dysregulated host response to infection” [
10,
11].Severe sepsis was defined as an infection related to one or more organ dysfunctions within 24 hours of admission [
12]. To evaluate a suspected case of sepsis, qSOFA criteria were used [
10,
11]. The presence of 2 or more parameters of the criteria is considered as a strong indicator of sepsis.
Eighty-five subjects with PAS were enrolled during this period. Sixteen subjects died; thus, the mortality rate was 18.82%. A detailed history and clinical examination were performed for all patients with PAS. PCT levels along with other laboratory parameters were investigated at admission and subsequently at 48 hours. Blood, high vaginal swab, urine, and pus (if present) samples were obtained and sent for bacterial culture and sensitivity. Requisite imaging was also performed. Key body systems (pulmonary, cardiac, renal, hepatobiliary, and neurological) were regularly assessed for signs of organ failure, and if detected, were managed accordingly.
Patients were classified into 2 groups based on organ failure: non-severe PAS, when there was no organ failure within 24 hours of admission and severe PAS when ≥1 organ failure occurred within 24 hours of admission [
12]. Patients were further managed according to the hospital protocol and the individual patient’s condition. PCT quantitative analysis was performed using the human PCT enzyme-linked immunosorbent assay (Biovendor Research and Diagnostic Products
©, Brno, Czech Republic).
4. Statistical analysis
The statistical tests were performed using the online software ‘
www.socscistatistics.com’. PCT levels were recorded as interquartile ranges (IQRs) along with median values. All descriptive parameters are expressed as percentages for qualitative parameters and as mean±standard deviation for quantitative parameters. The patients were stratified into different groups according to number of organ failures, and mortality. Intergroup and intragroup PCT levels were compared using non-parametric tests. A
P-value of <0.05 was considered significant.
Go to :

Discussion
Most subjects with PAS were young and in the postpartum period (mean age 26 years; postpartum 55%). A large number of patients presented with organ failure (74%) at the time of admission as they were late referrals. Bacteria were isolated in 64% patients, based on culture. Almost 19% patients with PAS died despite critical care.
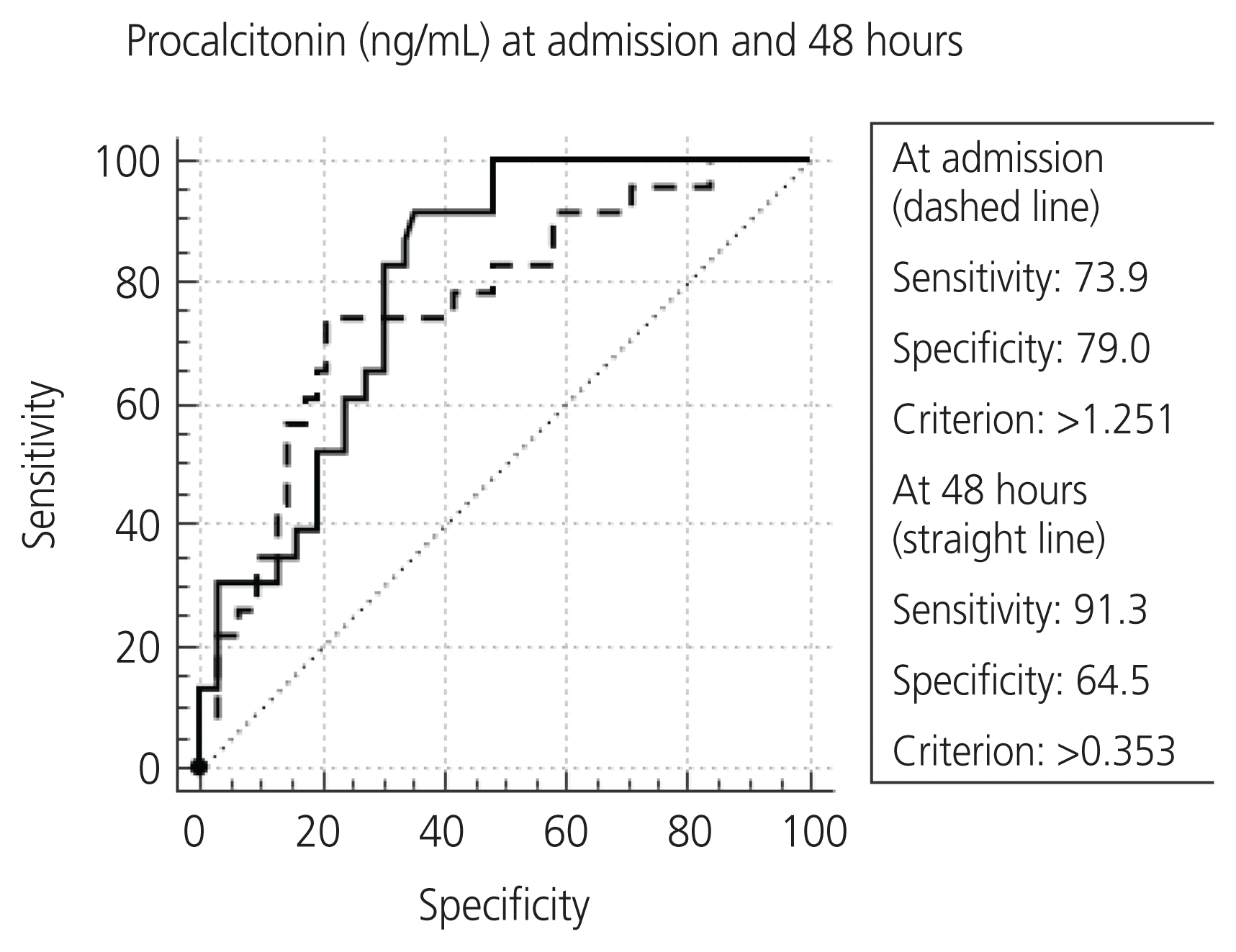
The overall median IQR PCT values at admission were higher in patients with severe PAS than in those without severe PAS. At 48 hours, this difference was statistically significant. Further, higher the number of organ failures, higher were the PCT levels at 48 hours. This indicates that organ failure is associated with widespread systemic dysfunctions, and this might be responsible for the elevated PCT levels. The PCT levels, however, did not change significantly following the standard treatment in any of the groups, although it did fall quantitatively in most groups. As mentioned above, several patients in our series were brought in an advanced state of sepsis, which might be one of the other reasons for unaltered PCT within a span of 48 hours.
The PCT levels were significantly higher in the mortality group than in the survivors at admission (8.31 ng/mL vs. 1.72 ng/mL), and this difference increased further at 48 hours (9.54 ng/mL vs. 1.37 ng/mL). Thus, a non-declining PCT at 48 hours is a critical situation and requires urgent attention from the treating clinician.
The study found quantitative cut-off values of PCT at admission and at 48 hours (PCT cutoff >1.251 ng/mL and >0.0895 ng/mL, respectively) that were indicative of a risk to maternal life. This can be used in conjunction with clinical findings and other investigations for urgent interventions.
The repeat 48-hour PCT levels have been used in patients with trauma and burn injuries, as prognostic markers for the severity of sepsis and mortality [
13,
14]. Literature related to the use of PCT in obstetric sepsis is limited. Paccolat et al. [
3] reported a PCT cutoff level of 0.25 ng/mL (specificity 91% on day 3 postpartum) to rule out infection during the perinatal period. This is similar to the threshold used to diagnose systemic infections and to guide the use of antibiotics in infected adult populations. Velasquez et al. [
7] determined the usefulness of PCT in pregnancy-associated sepsis and for differentiating between bacterial and other causes of infection. Their study included 98 pregnant patients with suspected infection, and 45 with no infection. They found that median the IQR PCT was significantly higher in those with bacterial-related PAS compared to that in non-infected patients. They proposed a cutoff PCT value of 0.06 ng/mL (sensitivity 60%; specificity 84%) for the diagnosis of bacterial sepsis. They concluded that PCT can be useful in complementing the clinical findings for the diagnosis of sepsis in pregnancy, in a reliable and timely manner. Another study by Agarwal et al. [
8] similarly found a significant difference between PCT levels in PAS cases and healthy controls. The study involved 40 cases and 40 controls. The mean PCT level in PAS cases was 2.46 ng/mL, while in controls it was 0.091 ng/mL. Additionally, mean PCT levels differed significantly between those with severe (3.12 ng/mL) and non-severe PAS (0.49 ng/mL) (
P=0.04).
There are variations in PCT levels in obstetric studies related to PAS [
3,
8,
9]. There are several physiological and hormonal changes during pregnancy that could also influence the decisive PCT levels for the diagnosis of sepsis. This might also be due to the different timings of sample collection and variations in the severity of sepsis in the studied populations. However, the rise in PCT levels in association with the severity of sepsis has been reported escribed in all the published studies. The current study further reiterates the finding that PCT levels are significantly different in severe PAS compared to those in non-severe PAS.
The high morbidity and mortality associated with PAS demands strengthening of health care at both, peripheral and tertiary levels. Many patients with severe sepsis follow a downhill course despite active organ support and critical care admission. Therefore, early recognition of sepsis in pregnant women is of paramount importance.
C-reactive protein is widely used in clinical practice as a nonspecific acute-phase indicator of inflammation. However, its use in PAS is limited. A wide range of C-reactive protein levels (40–200 mg/dL) are seen due to the physiological changes occurring during pregnancy; hence, they pose a diagnostic dilemma. Furthermore, the levels are increased in both, bacterial and non-bacterial inflammation [
15,
16].
Although the use of PCT for PAS is relatively new, favorable evidence is slowly emerging. A raised PCT level at admission and its persistence at 48 hours can be a warning sign of severe PAS. The levels are higher in cases with multiorgan failures. Thus, PCT can be useful for assessing the severity of systemic inflammation, the risk of organ dysfunction, and mortality in conjunction with clinical judgment.
PCT was originally developed for differentiating sepsis from a bacterial vs. non-bacterial etiology in adult patients. However, the indications gradually extended to guide and follow antibiotic management in sepsis, correlating with the severity of sepsis and as a biomarker of critical care admission. Currently, daily quantitative measurement of PCT is recommended in critically ill patients with a suspected diagnosis of systemic inflammation, to monitor the systemic inflammation and success of therapy [
1,
2]. This approach affects the therapeutic and diagnostic decisions, if PCT levels are interpreted along with other clinical data. The role of PCT in obstetric sepsis is far from established, in view of the limited literature on the subject.
Our study failed to find statistically significant changes in PCT levels over a span of 48 hours following standard treatment, which needs substantiation with further studies. Whether spacing the PCT testing at 72 hours or beyond could be useful to monitor the effects of the interventions remains to be seen in future studies. Determining the threshold values of PCT for the diagnosis of PAS remains a challenge.
Repeat sampling for PCT at 48 hours to determine the diagnosis for maternal outcomes is a new initiative in PAS. PCT levels correlate with the severity of systemic inflammation. Therefore, standalone estimation of PCT at 48 hours can also be helpful as a prognostic tool for the patients’ condition. The number of subjects in our study provided statistical strength.
Our research was conducted in a referral tertiary care obstetric center. There were more sick patients at admission. Thus, the results of our study might not be applicable for other setups and patient groups. Our study did not include healthy controls. The findings of this study should be interpreted with the following background. PCT continues to be investigated in several observational and interventional studies. It has been demonstrated to have good performance characteristics for the identification of bacterial infection and sepsis [
2,
17–
21]. However, the evidence on PCT guided antibiotic therapy is less forthcoming. A recent Cochrane review cited inadequate evidence for PCT based antibiotic decisions for patients with sepsis, severe sepsis, or septic shock [
22]. Further, the use of PCT for PAS is relatively new. In our study, the response to antibiotics and treatment was not monitored via PCT levels.
In conclusions, repeat PCT estimation at 48 hours could complement clinical findings and enhance its prognostic value for patients with PAS.
Go to :





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download