Abstract
Pancreatic candidiasis can develop in patients with acute pancreatitis, compromised immune responses, or iatrogenic intervention. This paper reports a case of pancreatic candidiasis presenting as a solid pancreatic mass in a patient without the risk factors. A previously healthy 37-year-old man visited the emergency department with left flank pain. Abdominal CT revealed a 5 cm, irregular heterogeneous enhancing mass accompanied by a left adrenal mass. Positron emission tomography-computed tomography and endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) could not discriminate pancreatic cancer from infectious disease. A laparoscopic exploration was performed for an accurate diagnosis. After distal pancreatectomy with splenectomy and left adrenalectomy, pancreatic candidiasis and adrenal cortical adenoma were diagnosed based on the pathology findings. His condition improved after the treatment with fluconazole. This paper reports a case of primary pancreatic candidiasis mimicking pancreatic cancer in an immunocompetent patient with a review of the relevant literature.
The incidence of fungal infections has increased significantly over the past 20 years. These infections are associated with the greater use of immunosuppressive agents, broadspectrum antibiotics, indwelling catheters, and acquired immunodeficiency syndrome.1 Invasive fungal infections have a higher fatality rate than bacterial sepsis.2 Pancreatic fungal infections appear to penetrate the pancreas from the gastrointestinal tract, where fungal organisms are normally present.3
Pancreatic candidiasis is the most common fungal infection. The normal pancreatic gland is relatively resistant to invasion by fungal organisms, but an inflamed gland, which is observed in severe acute pancreatitis, is more susceptible to Candida infections.4 Other causes reported in patients after ampullectomy3 include ERCP in acute pancreatitis,5 or postoperatively.6 On the other hand, pancreatic candidiasis in patients without these risk factors has never been reported.
A 37-year-old man presented with a pancreatic mass mimicking pancreatic cancer that was accompanied by a left adrenal mass on abdominal CT scan. He was diagnosed with pancreatic candidiasis after a surgical resection and was treated with an antifungal agent. This paper reports a case of pancreatic candidiasis that developed in an immunocompetent host with a review of the relevant literature.
A 37-year-old man visited the emergency room because of left flank pain. Two days prior, he visited a primary clinic for the same symptom and was diagnosed with a urinary tract infection. Despite being treated with antibiotics, his symptoms worsened, prompting his visit to the Chungbuk National University Hospital. His history included hypertension and weight loss of 6 kg for 3 months, and his family history was not specific. His social history of alcohol consumption and smoking was unremarkable. His BMI was 22.6 kg/mm2 (172.2 cm in height and 67.1 kg in body weight). The initial vital signs were a blood pressure of 137/77 mmHg, a heart rate of 96 beats per min, a respiratory rate of 18 breaths per min, and a body temperature of 37.0℃. An abdominal physical examination showed normal bowel sounds and mild tenderness in the left upper quadrant of the abdomen.
The laboratory tests revealed the following: a white blood cell count of 24,300/mm3 (neutrophil 85.6%, lymphocyte 7.9%, monocyte 4.8%, and eosinophil 1.2%); hemoglobin of 11.9 g/dL, platelet count of 586,000/mm3; PT of 11.7 sec; activated partial thromboplastin time of 31.2 sec; BUN of 28.3 mg/dL; creatinine of 2.37 mg/dL; protein of 7.7 g/dL; albumin of 4.2 g/dL; AST of 20 IU; ALT of 13 IU/L; ALP of 126 U/L; total bilirubin of 0.28 mg/dL; amylase of 56 IU/L; lipase of 38 IU/L; CRP of 18.31 mg/dL (range 0-0.3 mg/dL); CEA of 3.27 ng/mL (range 0-5 ng/mL); CA 19-9 of 292.24 U/mL (range 0-37 U/mL); AFP of 5.77 ng/mL (range 0.89-8.78 ng/mL); and negative HBsAg, anti-HBsAb, anti-HCV, and an-ti-HIV. The other blood and urine tests were normal.
Chest and abdominal radiography revealed nonspecific findings. Abdominal CT showed a 4.5×4.3 cm irregular heterogeneous enhancing mass in the tail of the pancreas with internal necrosis. A heterogeneously enhancing mass in the left adrenal gland and multiple lymphadenopathies, approximately 1.0 cm in size, was observed (Fig. 1A). Pancreatic cancer with cystic degeneration and left adrenal gland metastasis was suspected. PET-CT was performed to evaluate the tumors. The PET-CT scan revealed a hypermetabolic mass at adrenal mass for metastatic masses or double-primary the pancreatic tail (SUVmax 10.8) and a low attenuated mass showing minimal uptake at the left adrenal gland (SUVmax 5.0), indicating a benign neoplasm (Fig. 1B).
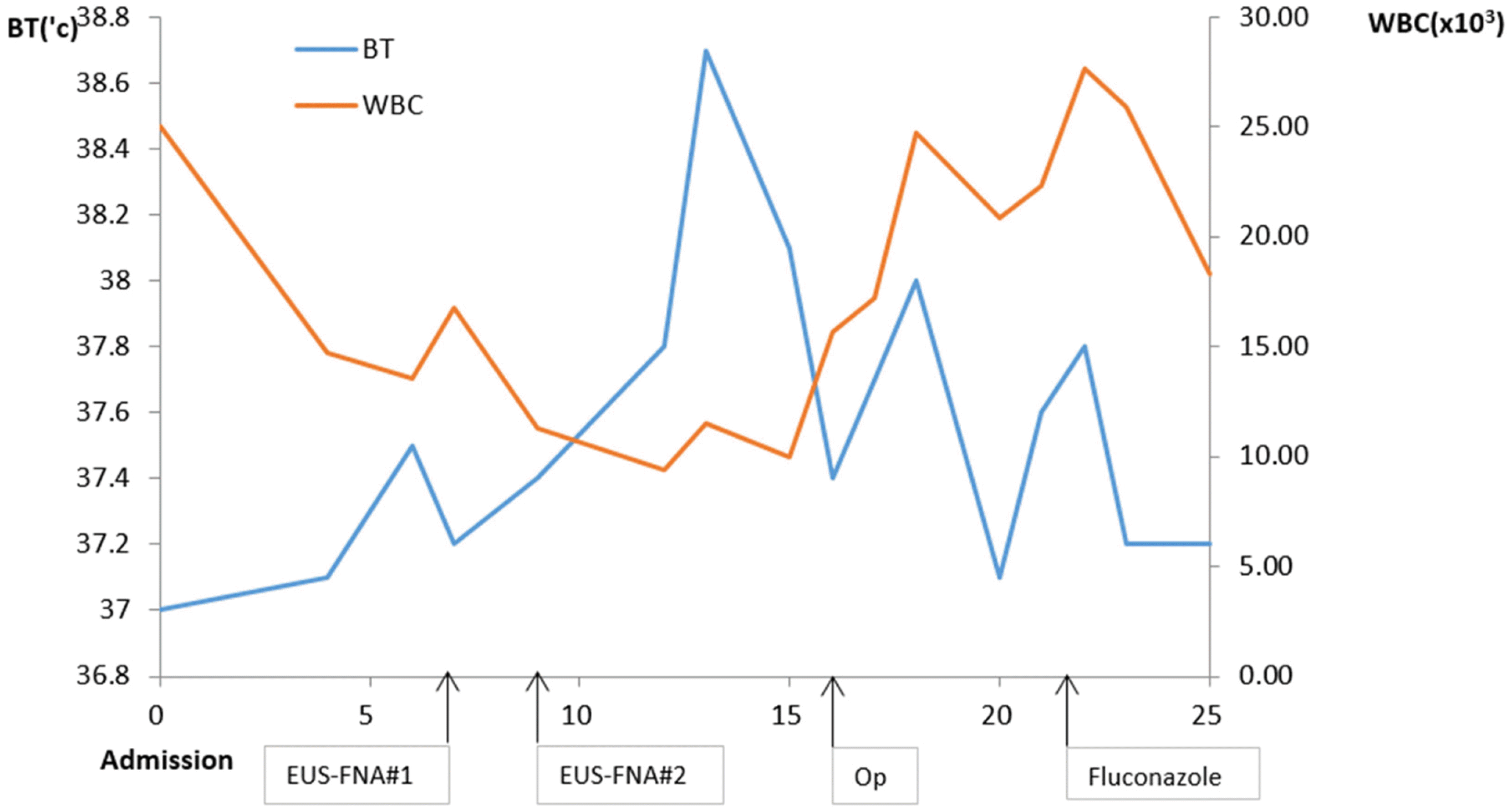
After admission, high fever persisted during hospitalization despite the administration of parenteral broad-spectrum antibiotics, namely cefotaxime 2.0 g and metronidazole 0.5 g 3 times a day. For a differential diagnosis of a pancreatic abscess and pancreatic cancer, endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) using a 22G needle (Echotip; Wilson-Cook, Winston Salem, NC, USA) was conducted using a linear EUS (GF-UCT240-AL5; Olympus, Tokyo, Japan). EUS showed an ill-defined heterogeneous hypoechoic mass at the pancreatic tail (Fig. 1C). EUS-guided FNA revealed only inflammatory cells, with neither malignant cells nor pathogenic organisms noted (Fig. 1D). The follow-up laboratory data revealed normal levels of BUN and creatinine. All laboratory data indicating pheochromocytoma for the left adrenal mass were negative. In addition, his high blood pressure was diagnosed as primary hypertension, owing to the negative results for all possible causes of secondary hypertension.
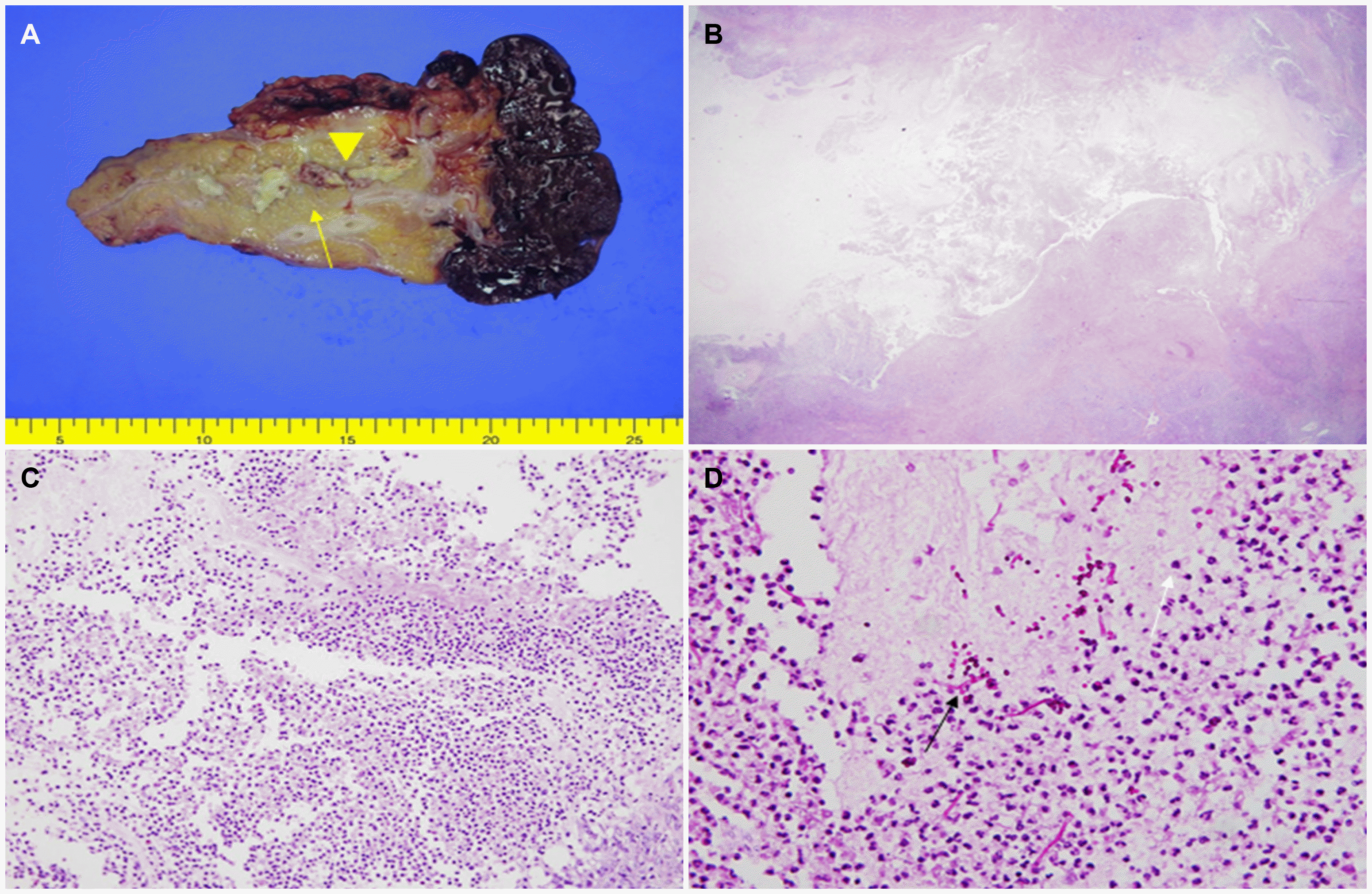
A laparoscopic exploration was performed for an accurate diagnosis. Distal pancreatectomy with splenectomy and left adrenalectomy were performed. The hard mass was resected from the tail of the pancreas, and the mass showed severe adhesions around the pancreas. A 2 cm mass was also resected in the left adrenal gland. The gross findings of the pancreatic mass were yellow necrotic substances in the center surrounded by lobulated pancreatic parenchyma. The microscopic findings showed necrosis, severe inflammation by neutrophils and hyphae, and yeast by PAS staining, indicating pancreatic candidiasis (Fig. 2). The mass on the left adrenal gland was diagnosed as an adrenal cortical adenoma, and the multiple lymph nodes were diagnosed as reactive changes. The patient was treated with 400 mg parenteral fluconazole once a day for 7 days, which was then changed to the oral form for an additional 2 weeks (Fig. 3). At 2 months, his symptoms improved, and the CA 19-9 level had returned to normal at 4.56 U/mL. He was followed up for 1 year without symptoms.
Pancreatic fungal infections occur in patients with severe acute pancreatitis,7 a history of surgical manipulation of the abdomen after an episode of acute pancreatitis, ERCP,8 total parenteral nutrition, and mechanical ventilation.9 An altered host immune system due to chemotherapy, steroid therapy, comorbid diabetes mellitus, and advanced age is also a predisposing risk factor for pancreatic fungal infections.10 Compared to mild pancreatitis, severe pancreatitis is an immunocompromised state with fewer major histocompatibility complex (class I antigen)-expressing cells and CD4+ cells.11 Broad-spectrum antibiotic therapy for pancreatic necrosis alters the intestinal flora and facilitates fungal invasion of the pancreas.12 Chronic indwelling devices can be the entry pathway for invasive fungi or colonized by fungal pathogens.10
The early diagnosis of pancreatic candidiasis is necessary to improve its prognosis. On the other hand, it is difficult to distinguish it from bacterial infections by its clinical symptoms alone. The imaging characteristics of pancreatic candidiasis include a cystic mass with a peripheral enhanced portion and inner necrotic portion. On the other hand, it can present as a solitary abscess,3 mimicking pancreatic cancer,13 and cystic tumors.3 One study reported magnetic resonance images that revealed restricted diffusion on a background of pancreatitis.3 The present case presented as a heterogeneous solid mass with focal necrosis mimicking pancreatic cancer.
A definite diagnosis of pancreatic candidiasis can be made by the microbiological isolation of Candida from pancreatic tissue. The diagnosis is termed primary when a positive smear or culture is obtained during initial radiological/endoscopic/surgical intervention and secondary when it occurs after a prior intervention. In this case, the patient was diagnosed with primary pancreatic candidiasis, as he had never been treated before. Candida species (yeasts) are fungal organisms that present in a unicellular form and form both pseudohyphae and hyphae.10 EUS-FNA is a useful tool for tissue acquisition from the adrenal gland,14 mediastinum,15 or pancreas 16 for the isolation of fungal organisms. In this case, the patient was suspected of having infectious diseases despite the high CA 19-9 and solid mass on imaging findings. On the other hand, EUS-FNA revealed only acute inflammatory cells without organism identification. In this case, the number of Candida was too sparse to be identified in the aspirate material. Repeated EUS-FNA with culture or EUS guided fine needle biopsy (EUS-FNB) could be an alternative to a surgical resection. Recent meta-analysis revealed EUS-FNB to have higher diagnostic accuracy than EUS-FNA for solid pancreatic masses.17
This case revealed a transient elevation of CA 19-9. Although a high CA 19-9 level is a useful marker of malignant pancreaticobiliary diseases,18 it has been reported in benign conditions, such as choledocholithiasis, biliary obstruction, pancreatitis, and pancreatic cysts.19
Systemic antifungal therapy should be initiated early in disease progression.9 In acute necrotizing pancreatitis, anti-fungal therapy should be initiated when fungi are identified in the pancreatic tissue. Fluconazole or amphotericin B should be used as antifungal therapy. Fluconazole may be an excellent alternative because intravenous amphotericin B is associated with severe nephrotoxicity.9 A randomized study comparing fluconazole and amphotericin B reported an equivalent effect when administered to non-neutropenic patients with candida infection.20
This patient presented with pancreatic cancer according to imaging findings, high CA 19-9 levels, and weight loss, but his young age, fever, and negative cytology were incompatible with a malignancy. The combined adrenal mass was considered to be a metastatic lesion on the initial CT scan. On the other hand, the adrenal gland is not a common metastatic site of pancreatic cancer, and its SUVmax is lower than that of a pancreatic lesion. Therefore, it was suggested to be an incidental lesion. His diagnosis was unexpected because he was immune-competent and had never received invasive interventions. Pancreatic fungal infections, particularly Candida infections, have attracted more attention because of the increasing incidence and high mortality rate. This paper reports pancreatic candidiasis in a patient without the risk factors with a review of the relevant literature.
REFERENCES
1. Ascioglu S, Rex JH, de Pauw B, et al. 2002; Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis. 34:7–14. DOI: 10.1086/323335. PMID: 11731939.


2. Lin SJ, Schranz J, Teutsch SM. 2001; Aspergillosis case-fatality rate: systematic review of the literature. Clin Infect Dis. 32:358–366. DOI: 10.1086/318483. PMID: 11170942.


3. Seong M, Kang TW, Ha SY. 2015; Pancreatic candidiasis that mimics a malignant pancreatic cystic tumor on magnetic resonance imaging: a case report in an immunocompetent patient. Korean J Radiol. 16:1253–1256. DOI: 10.3348/kjr.2015.16.6.1253. PMID: 26576113. PMCID: PMC4644745.



4. Simonova J, Cuchrac L, Firment J, Takacova V, Vasko L, Vaskova J. 2017; Invasive yeast infection in patient with acute pancreatitis. Imaging J Clin Medical Sci. 4:6–10.

5. Jalan R, Jones HL, Walker RJ. 1994; Multiple pancreatic abscesses due to Candida albicans following ERCP. Scott Med J. 39:17–18. DOI: 10.1177/003693309403900105. PMID: 8720751.


6. Howard JM, Bieluch VM. 1989; Pancreatic abscess secondary to Candida albicans. Pancreas. 4:120–122. DOI: 10.1097/00006676-198902000-00019. PMID: 2717601.


7. Baronia AK, Azim A, Ahmed A, et al. 2017; Invasive candidiasis in severe acute pancreatitis: experience from a tertiary care teaching hospital. Indian J Crit Care Med. 21:40–45. DOI: 10.4103/0972-5229.198325. PMID: 28197050. PMCID: PMC5278589.


8. Hasan S, Fearn R. 2018; Fungal liver abscess in an immunocompetent patient who underwent repeated ERCPs and subtotal cholecystectomy. BMJ Case Rep. 2018:bcr2017222013. DOI: 10.1136/bcr-2017-222013. PMID: 29449266. PMCID: PMC5836631.

9. Kochhar R, Noor MT, Wig J. 2011; Fungal infections in severe acute pancreatitis. J Gastroenterol Hepatol. 26:952–959. DOI: 10.1111/j.1440-1746.2011.06685.x. PMID: 21299617.


10. Shanmugam N, Isenmann R, Barkin JS, Beger HG. 2003; Pancreatic fungal infection. Pancreas. 27:133–138. DOI: 10.1097/00006676-200308000-00005. PMID: 12883261.


11. Curley PJ, McMahon MJ, Lancaster F, et al. 1993; Reduction in circulating levels of CD4-positive lymphocytes in acute pancreatitis: relationship to endotoxin, interleukin 6 and disease severity. Br J Surg. 80:1312–1315. DOI: 10.1002/bjs.1800801031. PMID: 7902182.


12. Isenmann R, Schwarz M, Rau B, Trautmann M, Schober W, Beger HG. 2002; Characteristics of infection with Candida species in patients with necrotizing pancreatitis. World J Surg. 26:372–376. DOI: 10.1007/s00268-001-0146-9. PMID: 11865377.

13. Mannell A, Obers V. 1990; Pancreatic candidiasis. A case report. S Afr J Surg. 28:26–27. PMID: 2339303.

14. Eloubeidi MA, Luz LP, Crowe DR, Snowden C, Morgan DE, Arnoletti PJ. 2010; Bilateral adrenal gland enlargement secondary to histoplasmosis mimicking adrenal metastases: diagnosis with EUS-guided FNA. Diagn Cytopathol. 38:357–359. DOI: 10.1002/dc.21210. PMID: 19894255.


15. Yachimski P, Forcione D, Faquin W. 2007; Mediastinal cryptococcal abscess diagnosed by EUS-FNA. Gastrointest Endosc. 66:1023–1024. DOI: 10.1016/j.gie.2007.06.010. PMID: 17719038.


16. Tange K, Yokota T, Sunago K, et al. 2019; A rare case of acute pancreatitis caused by Candida Albicans. Clin J Gastroenterol. 12:82–87. DOI: 10.1007/s12328-018-0896-7. PMID: 30155835.


17. Li H, Li W, Zhou QY, Fan B. 2018; Fine needle biopsy is superior to fine needle aspiration in endoscopic ultrasound guided sampling of pancreatic masses: a meta-analysis of randomized controlled trials. Medicine (Baltimore). 97:e0207. DOI: 10.1097/MD.0000000000010207. PMID: 29595661. PMCID: PMC5895392.
18. Morris-Stiff G, Teli M, Jardine N, Puntis MC. 2009; CA 19-9 antigen levels can distinguish between benign and malignant pancreaticobiliary disease. Hepatobiliary Pancreat Dis Int. 8:620–626. PMID: 20007080.

19. Ito S, Gejyo F. 1999; Elevation of serum CA 19-9 levels in benign diseases. Intern Med. 38:840–841. DOI: 10.2169/internalmedicine.38.840. PMID: 10563741.

20. Robbins EG 2nd, Stollman NH, Bierman P, Grauer L, Barkin JS. 1996; Pancreatic fungal infections: a case report and review of the literature. Pancreas. 12:308–312. DOI: 10.1097/00006676-199604000-00016. PMID: 8830340.

Fig. 1
Pancreatic candidiasis in 37-year-old man. (A) Axial computed tomography (CT) scan reveals a 4.5×4.3 cm sized irregular heterogenous enhancing mass (arrow heads) with internal necrosis (asterisk) in the tail of pancreas. A heterogenous enhancing mass was also seen in the left adrenal gland (arrow). (B) Positron emission tomography-CT (PET-CT) shows a hypermetabolic mass at the pancreas tail (SUVmax 10.8, arrow heads) and a low attenuated mass showing minimal uptake at the left adrenal gland (SUVmax 5.0, arrow). (C) Endoscopic ultrasonography (EUS) shows an ill-defined heterogenous hypoechoic mass at the pancreas tail. (D) EUS-guided fine-needle aspiration was performed.
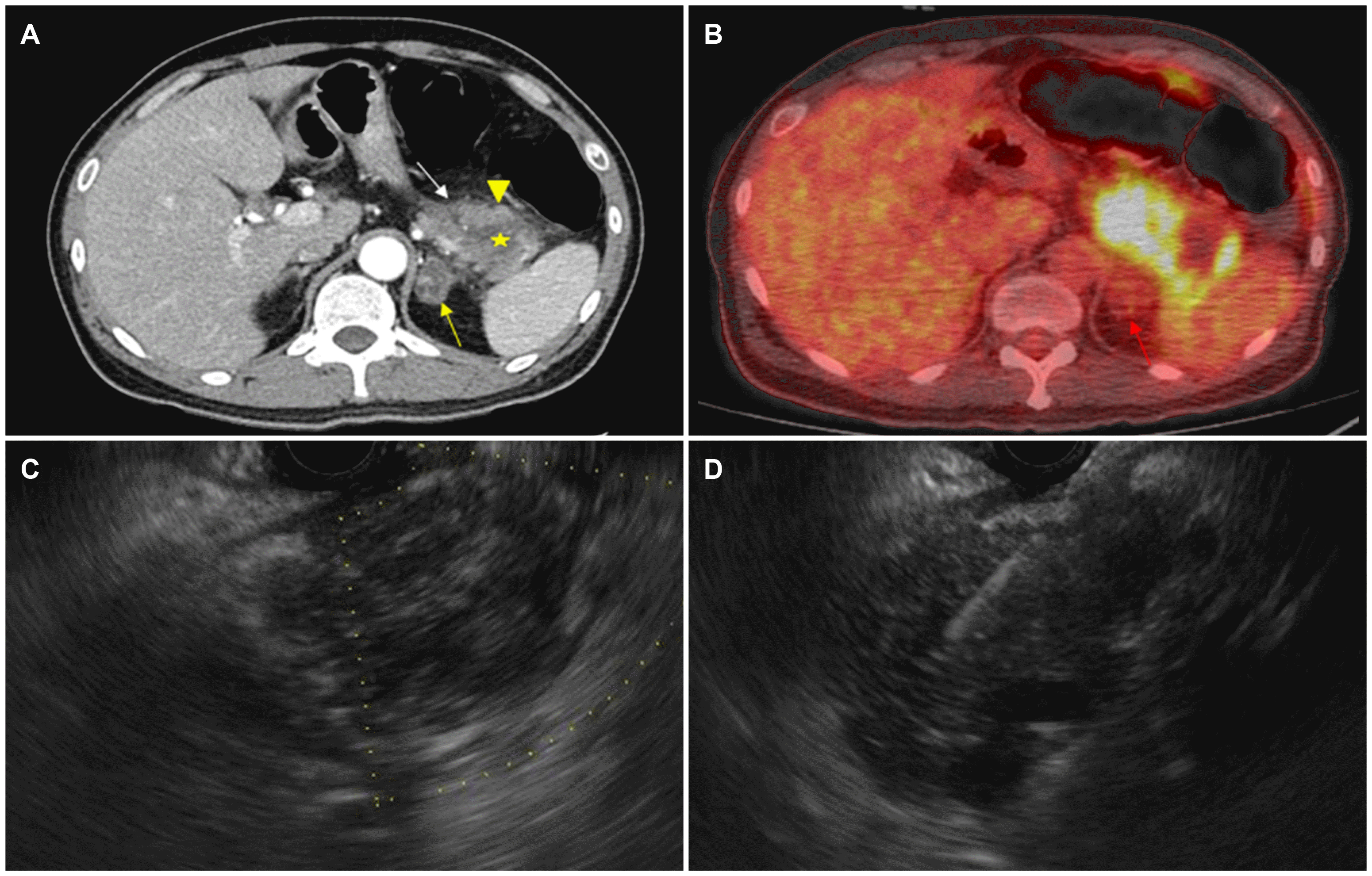
Fig. 2
(A) Gross findings of pancreatic mass show that there are necrotic substances in the middle (arrow head), and lobulation of the surrounding pancreatic parenchyma (arrow) is well maintained. (B-D) Microscopic findings. (B) Necrotic substances and severe inflammation can be seen at low magnification (H&E, ×12.5). (C) Most of necrotic substances were neutrophils at medium magnification (H&E, ×100). (D) Hyphae (black arrows) and yeast types (white arrows) were observed at high magnification, consistent with Candida species (PAS staining, ×200).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download