INTRODUCTION
Fasciola hepatica infections previously occur mainly in Western Europe, Central and South America, and the Middle East, but they are now widespread across all continents.
1,2 Several cases of human
F. hepatica infection have been reported in Korea.
3-5 Fascioliasis is a zoonotic disease caused by
F. hepatica, a flat, leaf-shaped liver fluke that usually infects cattle, sheep, and goats. The clinical signs and symptoms of fascioliasis usually include fever, abdominal pain, hepatomegaly, and abnormal liver function test results with peripheral eosinophilia.
6,7 In non-endemic regions, the diagnosis of an
F. hepatica infection is usually delayed because it is uncommon, and its symptoms are similar to those of other infectious diseases. Therefore, the diagnosis of an
F. hepatica infection should be considered to reduce complications in patients with fever, abdominal pain, hepatomegaly, and eosinophilia.
The authors recently encountered two cases of F. hepatica infection that were diagnosed serologically and treated successfully. This paper recognizes neglected diseases and increases the knowledge of fascioliasis.
Go to :

DISCUSSION
Human fascioliasis is usually caused by the ingestion of aquatic plants, such as watercress, or by drinking water contaminated with metacercariae. The disease can also be contracted by eating the uncooked liver of infected animals.
2 Human fascioliasis has a clinical course consisting of an acute hepatic phase and a chronic biliary phase.
2,6 The hepatic phase generally begins 4-12 weeks after metacercariae ingestion. After humans acquire the parasite, the infected metacercariae reach the duodenum, penetrate the intestinal wall, enter the abdominal cavity, penetrate the liver capsule, and invade the liver parenchyma. After approximately 6 weeks, the trematode invades the bile duct system.
6 In the hepatic phase, the clinical symptoms include fever, abdominal pain, nausea, vomiting, urticaria, diarrhea, anemia, and hepatomegaly. The laboratory tests usually reveal peripheral eosinophilia and abnormal liver function test results.
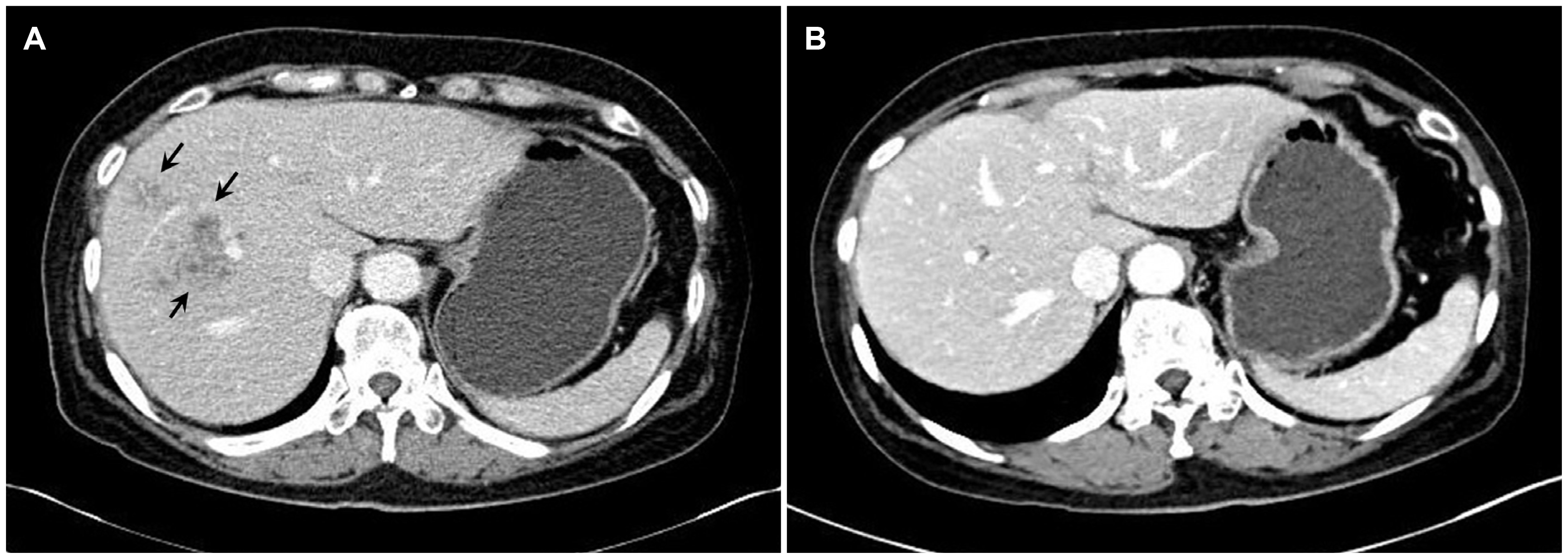
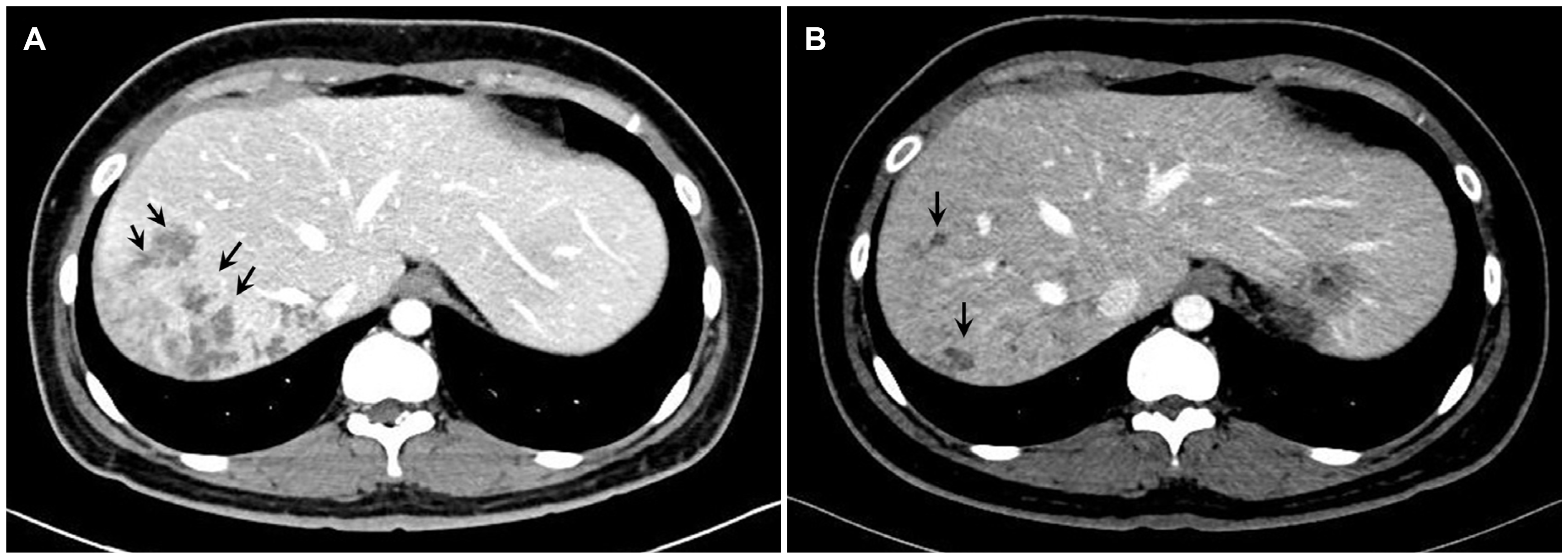
6,8 Typical enhanced CT findings in the acute phase include multiple, round, or oval clustered hypodense lesions with peripheral enhancement.
9 On the other hand, fascioliasis is difficult to differentiate from other causes of liver abscesses, such as pyogenic or amoebic infections. In addition, physicians usually consider
Toxocariasis or
Clonorchis sinensis first in patients with peripheral eosinophilia and eosinophilic abscess of the liver. A diagnosis of fascioliasis is usually determined by the detection of eggs in the stool or duodenal aspirates. In the acute phase, however, eggs may not be visible in the stool or aspirates.
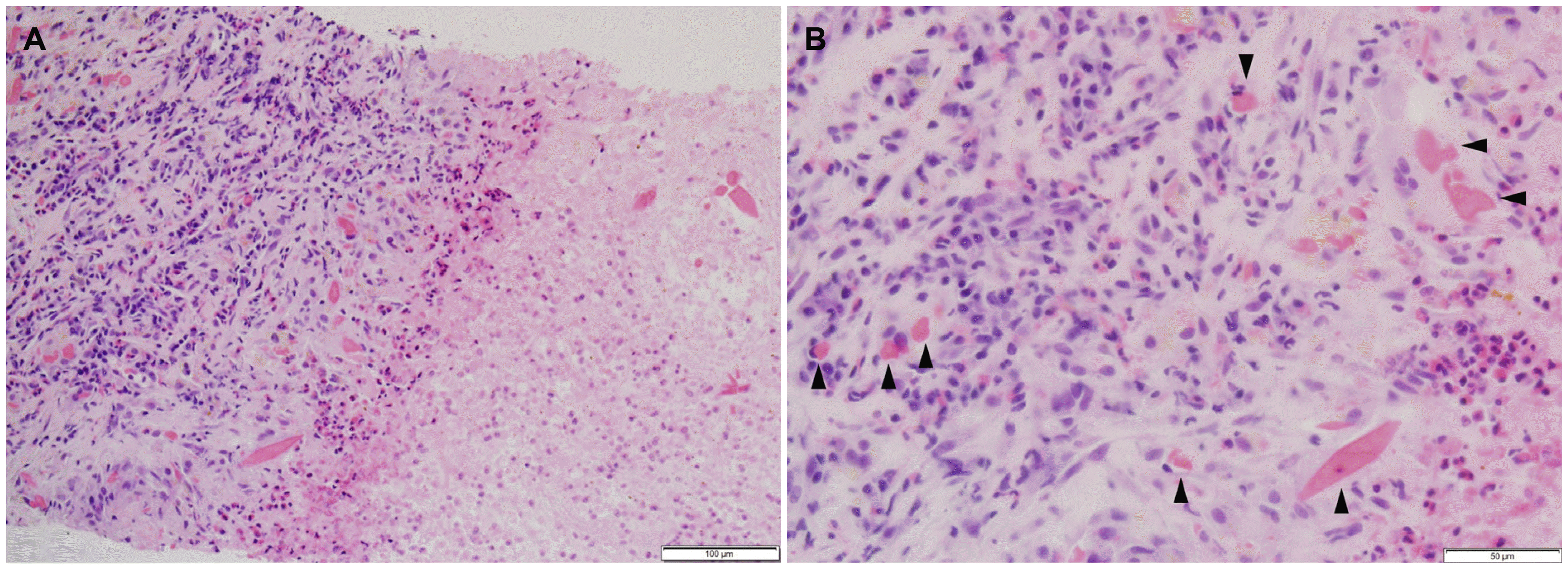
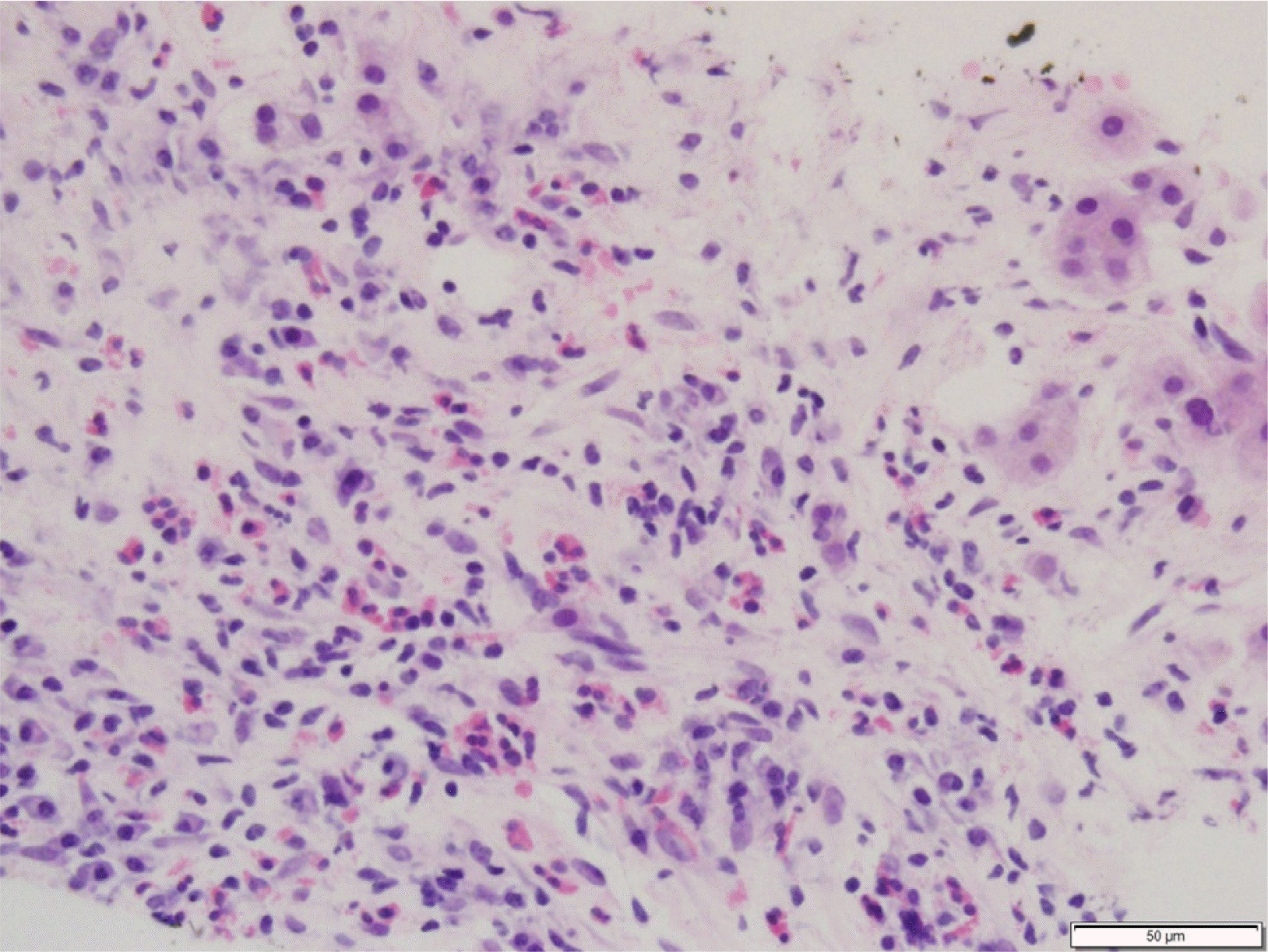
10 A liver biopsy is not usually performed, but the typical pathological findings of
F. hepatica include granulomatous inflammation with or without eggs, diffuse eosinophilic infiltration, migration track, Charcot-Leyden crystals, necrotic debris, and fibrosis.
11 Therefore, serologic tests, including ELISA, are required to diagnose fascioliasis in the acute phase. According to an endemic area study in Peru, ELISA revealed a sensitivity, specificity, and negative predictive value of 92.4%, 83.6%, and 97.2%, respectively.
12 In Korea, a serologic test by ELISA is available to refer to specialized agencies, and it takes approximately 10 days for the test result to be notified. The ELISA test for
F. hepatica is based on the antibody response method, and the test result is expressed as a numerical value.
12 The ELISA test is not perfect for distinguishing between overt and resolved infections; therefore, careful interpretation is required in clinical practice.
12
In the chronic biliary phase, patients usually present with epigastric or right upper quadrant pain, jaundice, intermittent fever, but approximately 50% of patients have no symptoms.
7 The laboratory tests show an elevation of total bilirubin, ALP, and GGT levels due to cholestasis. In particular, GGT begins to increase at 9 weeks after infection and increases significantly at 18 weeks. On the other hand, peripheral eosinophilia may not be observed in approximately 50% of patients because it could not be used for screening in endemic areas.
13 In the biliary phase, a diagnosis is based on a stool examination, serologic tests including ELISA, CT, and ERCP.
6,14 The detection of eggs in the stool, as well as in the duodenal and biliary aspirates is usually available at 10 weeks after infection.
2 The CT findings of the biliary phase reveal bile duct dilatation, irregular wall thickening, and low attenuation in the periportal areas. In some cases, multiple calcifications in the liver parenchyma may be observed.
9 ERCP may be helpful in a diagnosis if the biliary phase of the
F. hepatica infection is suspected. Flukes can be seen as a filling defect in the bile duct, and they can also be extracted by ERCP.
13,14
The two patients had atypical symptoms, such as dyspepsia, epigastric or chest discomfort, and a febrile sense with marked peripheral eosinophilia. The CT images revealed multiple clustered hypodense lesions and several ill-defined linear low densities in the liver. Infections with Toxocariasis or Clonorchis sinensis were first considered, but no eggs were revealed by stool examinations. Therefore, it was suspected that the patients were in the acute phase of the F. hepatica infection. The pathology findings of the liver biopsies supported the diagnosis of a parasitic infection, but F. hepatica could not be differentiated from other parasites. Ultimately, both patients were diagnosed definitively according to the serologic test results.
Praziquantel, the drug of choice for various trematodes, is ineffective on fascioliasis
15; rather, a single dose of triclabendazole (10 mg/kg) is recommended. Bithionol (30-50 mg/kg for 15 days) can also be effective.
7,16 Only eight well-organized cases of human fascioliasis were reviewed; all but one were treated with triclabendazole (
Table 1). In that case, nitazoxanide was prescribed because triclabendazole is unavailable in Nepal. In Korea, triclabendazole can be obtained only through the KOEDC, and it takes approximately 3-5 days to receive triclabendazole after submitting the required documents. In the present two patients, triclabendazole was administered successfully, and no adverse reactions or recurrence occurred.
Table 1
Demographics and Clinical Characteristics of the 94 Patients Included in the Study


In conclusion, the authors encountered two cases of acute-phase F. hepatica infection. Physicians should consider fascioliasis, particularly acute hepatic phase infection, in patients presenting with abdominal pain, marked peripheral eosinophilia, and hypodense hepatic lesions on CT. Serologic tests can help diagnose F. hepatica infections.
Go to :





 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download