CASE REPORT
A 14-year-old female patient was admitted to the gastro-enterology department with symptoms of abdominal pain, unintentional 6 kg weight loss over the past month, fatigue, and diarrhea with hematochezia. There was no history of contact with TB patients.
The laboratory results revealed hypochromic microcytic anemia, polymorphonuclear leukocytosis, thrombocytosis, and elevated CRP. The stool and urine microscopy results were normal. The fecal analysis results for bacterial cultures, parasites, and Clostridium difficile toxin were negative. Human immunodeficiency virus, HBV, HCV, and cytomegalovirus (CMV) serological test results were negative. The serological results were equivocal for IgG anti-Saccharomyces cerevisiae antibody (ASCA) (9.4; normal <7 units). The QuantiFERON gold assay result for a latent TB infection was negative. The liver and renal function tests results were normal.
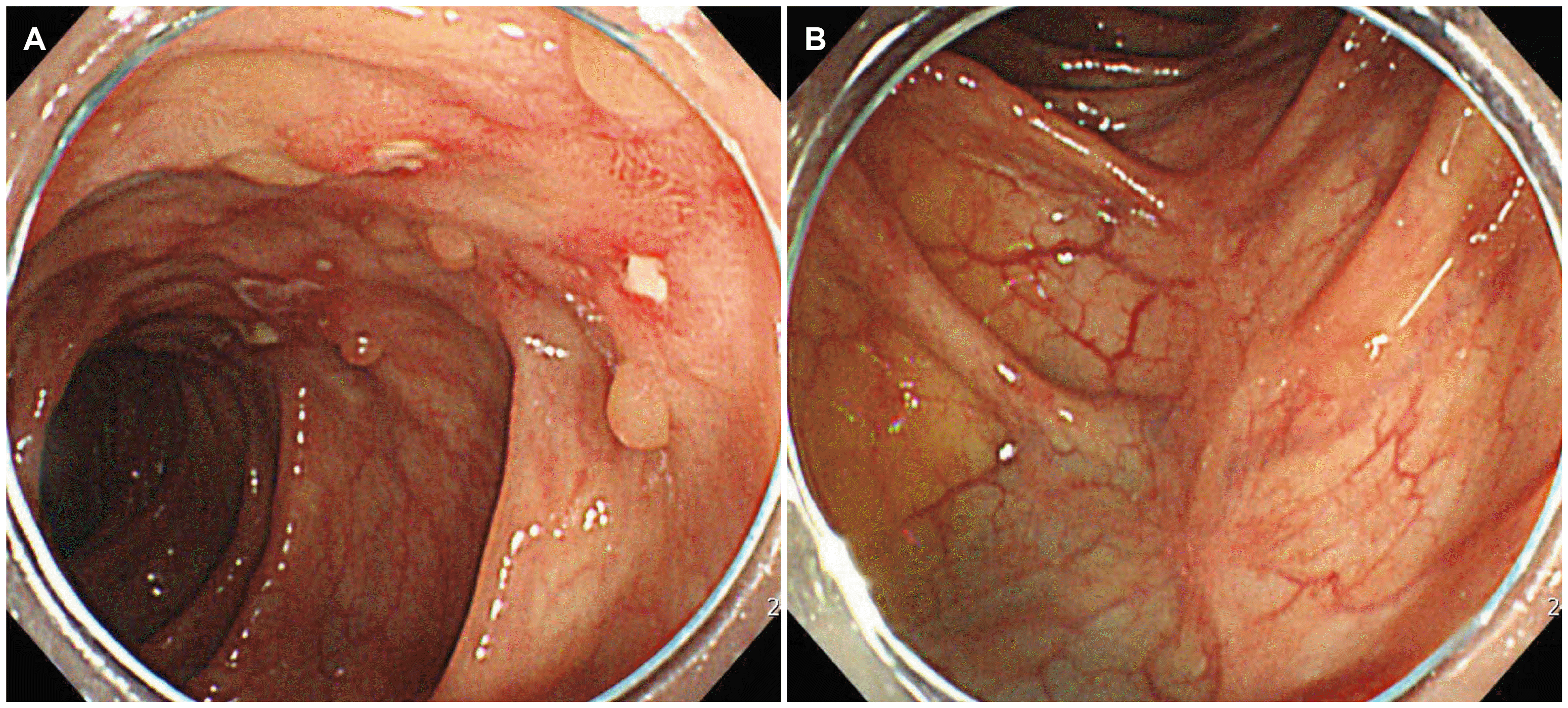
The patient underwent a colonoscopy that revealed a stricture of the ileocecal valve and scattered longitudinal ulcers in the whole colon, with the involvement of more than four segments (
Fig. 1). The pathology examination revealed chronic ulcerative inflammation. In addition, AFB staining of the intestinal tissue was negative. The CMV PCR and CMV immunostaining of the intestinal tissue were also negative.
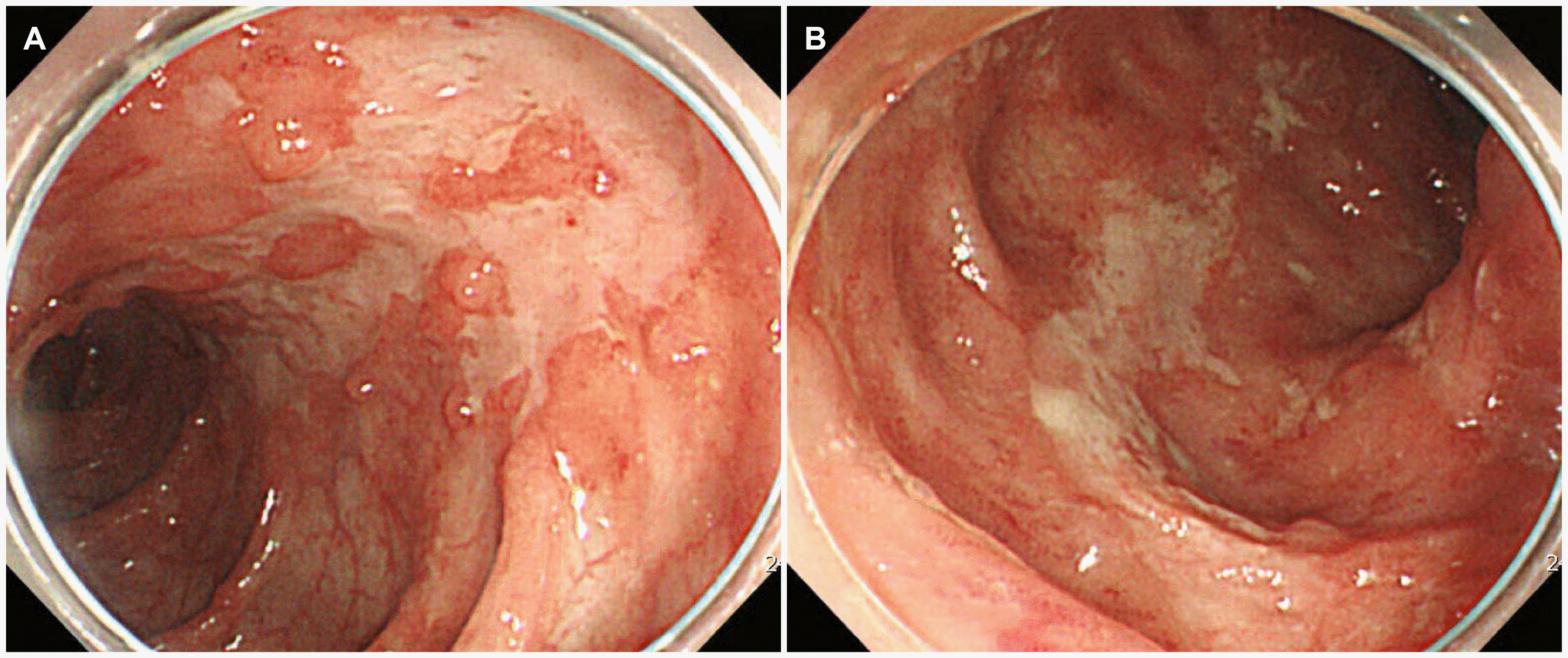 | Fig. 1Colonoscopy showing multiple scattered longitudinal ulcers and inflammation with mucosal hyperemia on the (A) ascending colon and (B) transverse colon. 
|
CT of the abdomen revealed a nonspecific colitis pattern of the whole colon and diffuse ascites (
Fig. 2). A chest CT scan was performed because the abdominal CT also showed branching nodular opacities and consolidation in the left lower lobe of the lung. The findings indicated active pulmonary TB (
Fig. 3). The chest x-ray showed consolidation in the left lower lobe of the lung (
Fig. 4A). Bronchoscopy was performed, and TB PCR of bronchial washing was positive.
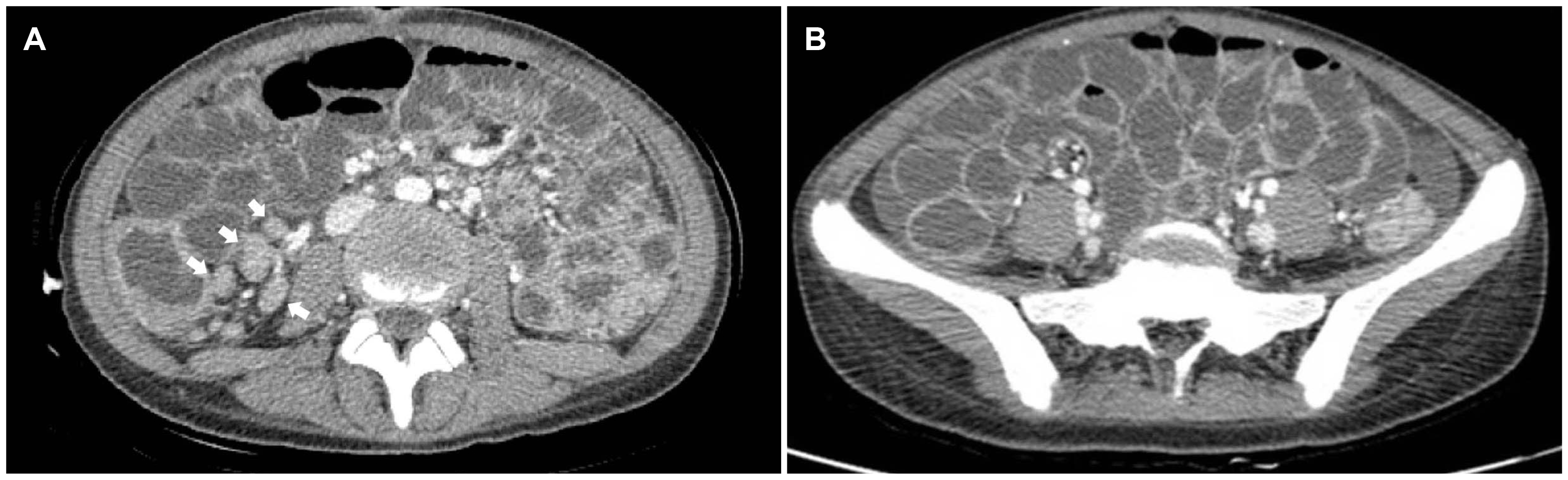 | Fig. 2Abdominal computed tomography scan revealing diffuse wall thickening of the whole colon and terminal ileum with several lymph nodes along the (A) ileocolic chain (arrows) and (B) ascites. 
|
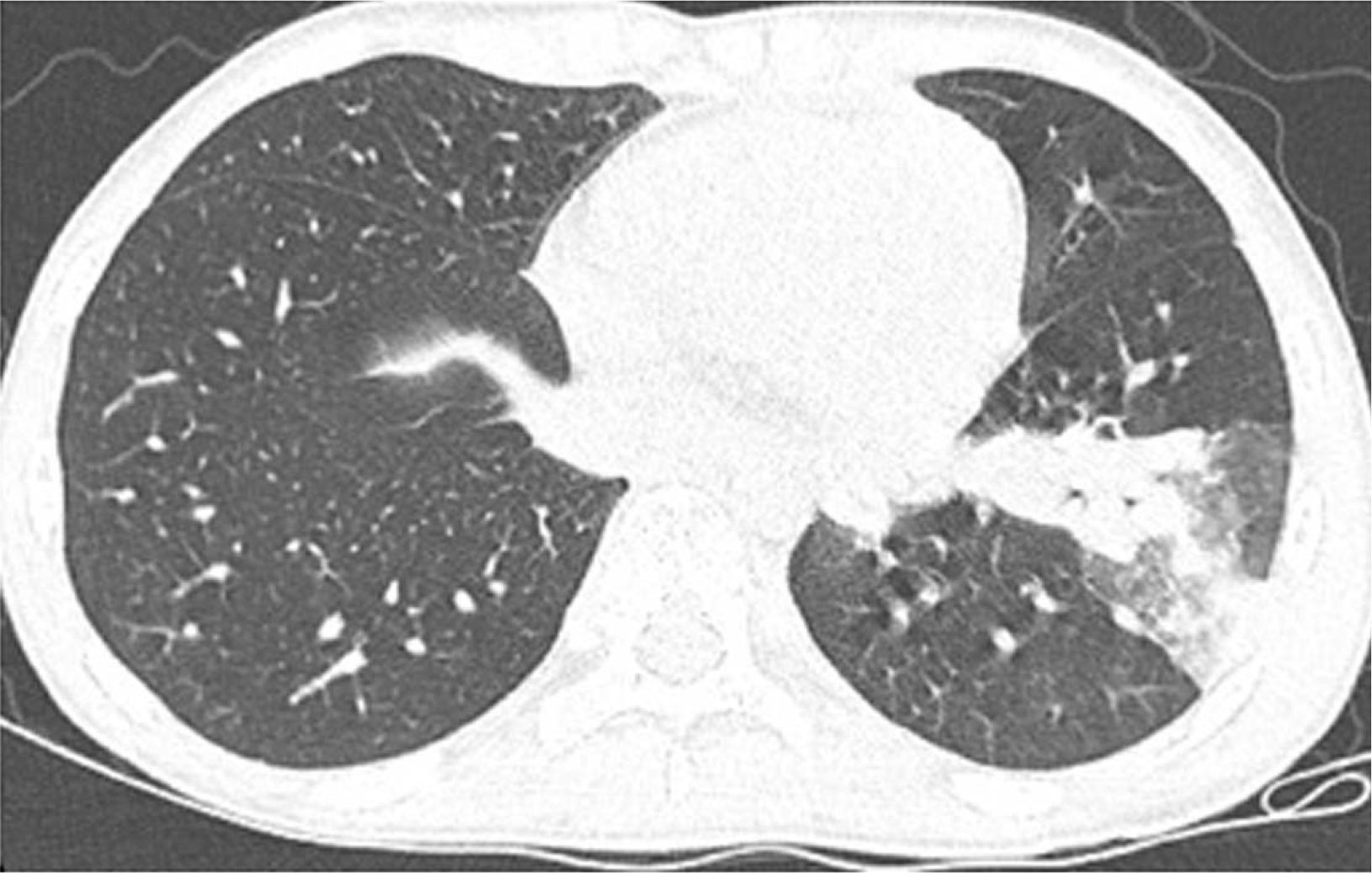 | Fig. 3Chest computed tomography scan showing branching nodular opacities and consolidation in the left lower lobe and left-sided pleural effusion. 
|
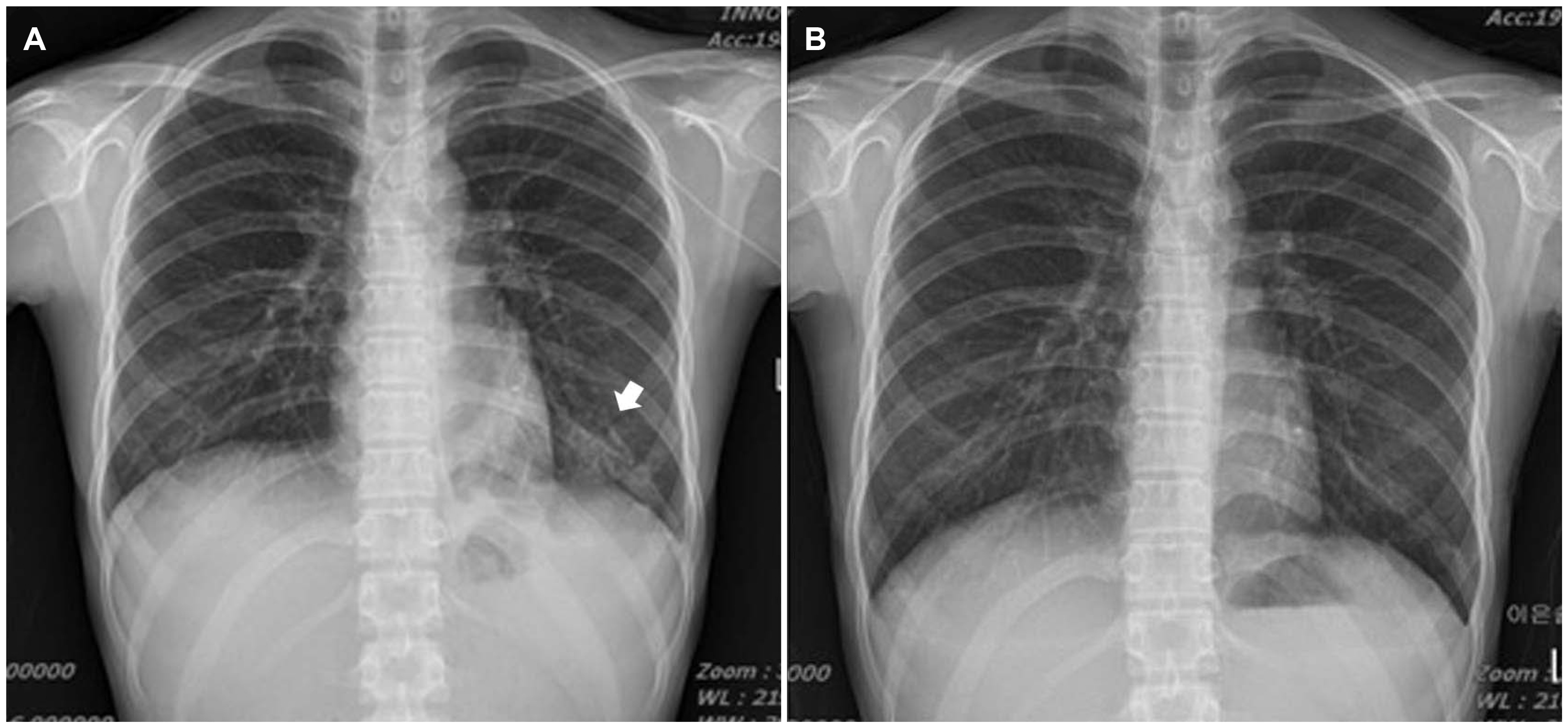 | Fig. 4Chest x-ray showing consolidation in the (A) left lower lobe (arrow) and (B) improvement of consolidation. 
|
The patient was diagnosed with pulmonary and intestinal TB. Although the colonoscopic findings favored CD, anti-tubercular therapy (ATT) was initiated based on the radiology findings and PCR test. Two weeks later, she presented with mild improvement of the abdominal pain, diarrhea, and hematochezia, but her gastrointestinal symptoms worsened over the next month despite the improvement observed on her chest X-ray (
Fig. 4B). She presented with worsening diarrhea and abdominal pain. The colonoscopy response was assessed after 8 weeks of ATT. Colonoscopy revealed exacerbated ulcers in the whole colon, and the stricture of the ileocecal valve persisted. Although the pulmonary TB had improved after ATT, the gastrointestinal symptoms were aggravated. Hence, the patient was diagnosed with CD and was treated with prednisolone, mesalazine, and ATT. She showed significant improvement in her clinical condition. A follow-up colonoscopy performed three months after treatment with prednisolone and mesalazine revealed considerable improvement of the ulcers (
Fig. 5).
 | Fig. 5Colonoscopy showing improvement of ulcers with healed scars on the (A) ascending colon and (B) transverse colon. 
|
After tapering the steroid therapy, the patient was treated with mesalazine and azathioprine. At the 10-month follow-up, the patient remained in clinical and biochemical remission.
Go to :

DISCUSSION
Approximately 33% of the world’s population has been exposed to TB, particularly in Southeast Asia, India, and Africa.
2 The disease demographics are changing due to immigration from endemic areas.
3 Of all TB cases, 10-12% of all TB cases are extrapulmonary TB; of these, 11-16% of cases involve the abdomen.
4 CD is a chronic inflammatory disease of an unknown etiology with possible genetic, environmental, and immunological factors.
1 Over the recent decades, the incidence of CD has increased in areas where the prevalence has been low.
CD is difficult to differentiate from intestinal TB because the clinical, endoscopic, and radiologic findings of these diseases are similar, particularly in areas where TB is endemic. In 2016, the incidence of TB in the Republic of Korea was 5.2 per 100,000 persons.
5 This incidence is the highest among member countries of the Organization for Economic Co-operation and Development. Distinguishing between intestinal TB and CD is critical because the treatments are radically different, and immunosuppressive medications for intestinal TB can be disastrous.
6
Endoscopy examinations, longitudinal ulcers, aphthous ulcers, cobblestone appearance, and anorectal lesions are more common in CD. Transverse ulcers, patulous ileocecal valve, and the involvement of fewer than four segments are more frequent in intestinal TB.
7 The endoscopy findings of the present patient were more suggestive of CD, but there were no reports of patients who presented with active pulmonary TB in the early stages of CD. Furthermore, immunosuppressive treatment can lead to the fatal spread of TB. Therefore, the patient was diagnosed initially with intestinal TB.
On a pathologic examination, multiple large caseating granulomas are found more frequently in intestinal TB, and trans-mural inflammation in the form of lymphoid aggregates is more common in CD. The presence of AFB in a biopsy on a histology examination is diagnostic of intestinal TB.
8
The CT findings more commonly show long-segment involvement, vascular engorgement of the mesentery, skip lesions, and symmetrical bowel wall thickening in CD.
9 Large necrotic lymph nodes (>1 cm) in the mesentery, ascites, and asymmetrical thickening of the colonic wall are features of intestinal TB.
10
Although extraintestinal manifestations are usually associated with CD, intestinal TB may also present with symptoms, such as uveitis and polyarthritis, mimicking extraintestinal CD.
11,12 The patient showed a pulmonary lesion. TB PCR of bronchial washing was positive, and bronchoscopy did not show inflammatory changes suggesting CD, including patches in the tracheobronchial system or redness. Therefore, the case was diagnosed as active pulmonary TB.
An interferon-gamma release assay was performed to confirm the latent TB infection. AFB staining and culture for
Mycobacterium TB of the patient’s tissue could also have been conducted, but these examinations show low diagnostic sensitivity. The QuantiFERON gold assay yielded negative results in this patient. Therefore, it was reasonable to attribute this result to the young age of the patient. False-negative QuantiFERON gold assay results are observed more often in younger persons.
13
ASCA is related to intestinal bowel disease. On the other hand, ASCA has limited value in the differentiation between CD and TB. Up to 65% of CD patients can present with ASCA, compared to 50% for intestinal TB.
14
Recent Asia-Pacific consensus statements for CD stated that in a patient with CD/intestinal TB dilemma, a diagnosis of CD should be considered in patients who do not respond to ATT and later respond to the treatment of CD. The clinical and colonoscopy response is assessed at 8-12 weeks of ATT. A diagnosis of intestinal TB is confirmed if the patient shows symptomatic improvement.
15 ATT can be considered in areas with the widespread prevalence of TB. Clinicians may consider ATT if a patient with the CD/intestinal TB dilemma presents with active pulmonary TB. On the other hand, unnecessary ATT may lead to a risk of hepatotoxicity, drug resistance, and a delay in the diagnosis of CD.
Physicians should integrate the clinical, endoscopy, and radiology findings to decide whether the diagnosis is intestinal TB or CD. Continuous caution in making a correct diagnosis based on sufficient findings after the initial diagnosis is very important. Clinicians should consider reevaluation, including repeating the CT scan, testing for TB, and colonoscopy.
Go to :


