INTRODUCTION
The primary goal of treating chronic hepatitis B (CHB) is preventing the progression of hepatic fibrosis by inhibiting the replication of the hepatitis B virus (HBV), stabilizing liver enzymes, and improving intrahepatic inflammation or fibrosis.
1-4 The longer-term goal is to inhibit the development of cirrhosis or liver cancer and improve the patient survival rates.
1-4 To date, approved antivirals for the CHB treatment include interferons and nucleos(t)ide analogs. The major drawback of a hepatitis B treatment using oral antiviral agents is the occurrence of antiviral resistance.
5
Lamivudine is the first approved drug for the treatment of CHB.
6 When resistance to lamivudine occurs, the treatment guidelines suggested switching to entecavir 1 mg or adding adefovir before tenofovir became widely available.
2,7,8 The same treatment is also applicable to the resistance to L-nucleoside analogs (telbivudine, clevudine) other than lamivudine.
5,9
Adefovir is a nucleotide analog drug that can be used in treatment-naïve or treatment-experienced patients. Combination therapy based on entecavir 1 mg is recommended if resistance to adefovir develops because these drugs do not share cross-resistance.
10 Recently, tenofovir monotherapy or a combination of tenofovir and entecavir 1 mg have been preferred.
1,3,4 Adefovir-or tenofovir-based treatment is recommended when entecavir resistance occurs, but entecavir 1 mg in combination is preferable to prevent further resistance.
11-13 In cases of multidrug-resistant CHB, a combination of tenofovir with entecavir 1 mg is recommended.
1,3,14,15 Therefore, entecavir 1 mg has frequently been prescribed as a mono-or combination therapy in various cases of resistance to existing antiviral agents.
With the recent expiration of the patent for entecavir, a variety of same-component products or generic drugs have become available in addition to the original drug, Baraclude® (Bristol-Myers Squibb [BMS], New York, NY, USA). On the other hand, clinical experience with these drugs is lacking. Moreover, the clinical equivalency of generic antiviral agents for CHB has not been demonstrated thus far, particularly in cases with previous antiviral resistance. Therefore, direct evidence regarding the actual clinical effects of generic drugs as replacements for Baraclude® 1 mg in CHB patients with antiviral resistance is needed.
This study examined the efficacy and safety of switching to a generic entecavir, Baracle® (Dong-A Science Technology [Dong-A ST], Seoul, Korea), 1 mg in CHB patients taking the brand-name entecavir, Baraclude®, 1 mg (BMS) alone or in combination with other agents for the treatment of resistance to nucleos(t)ide analogs.
Go to :

SUBJECTS AND METHODS
1. Ethics statement
The Institutional Review Board at Korea University Hospital approved the study protocol (IRB No. 2106AS0033). Written informed consent was obtained from all the patients, and the study was conducted in accordance with the ethical principles of the Declaration of Helsinki.
2. Study design
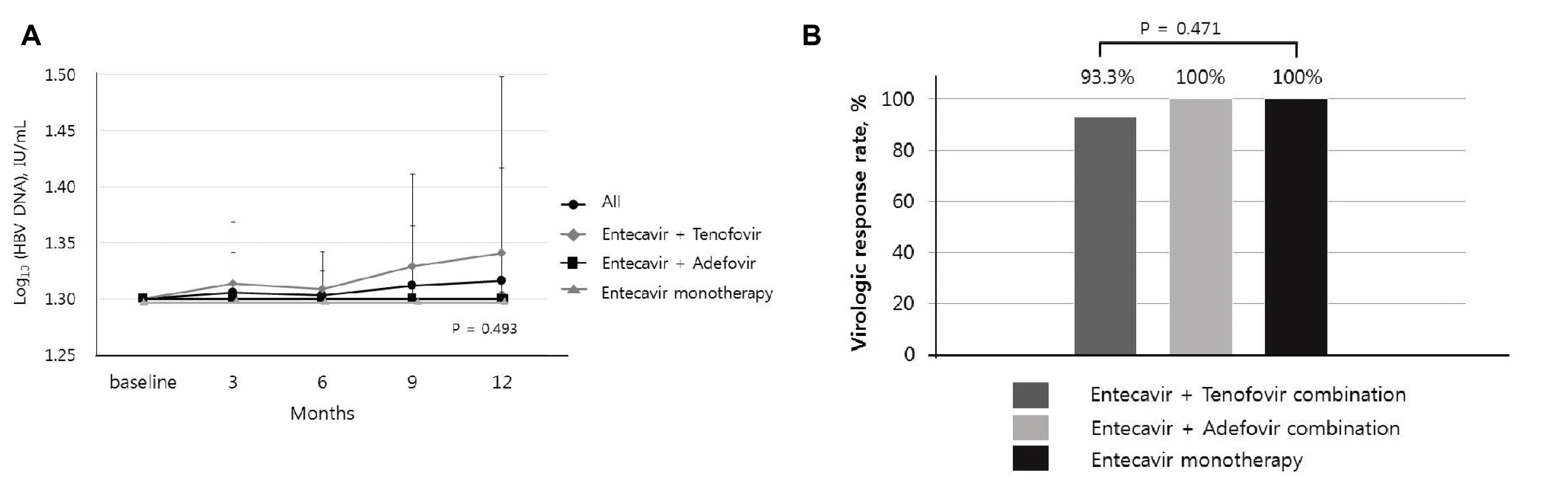
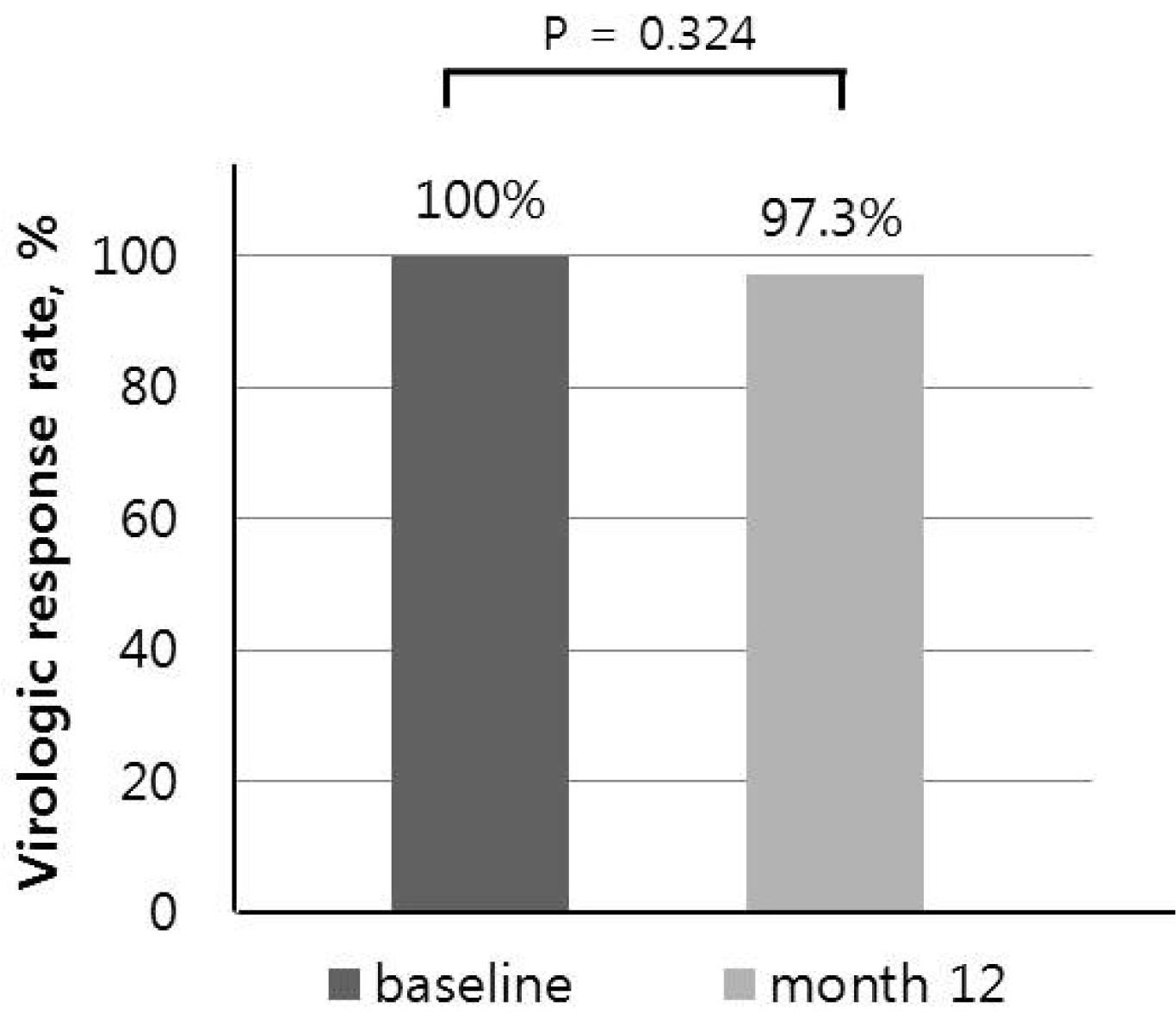
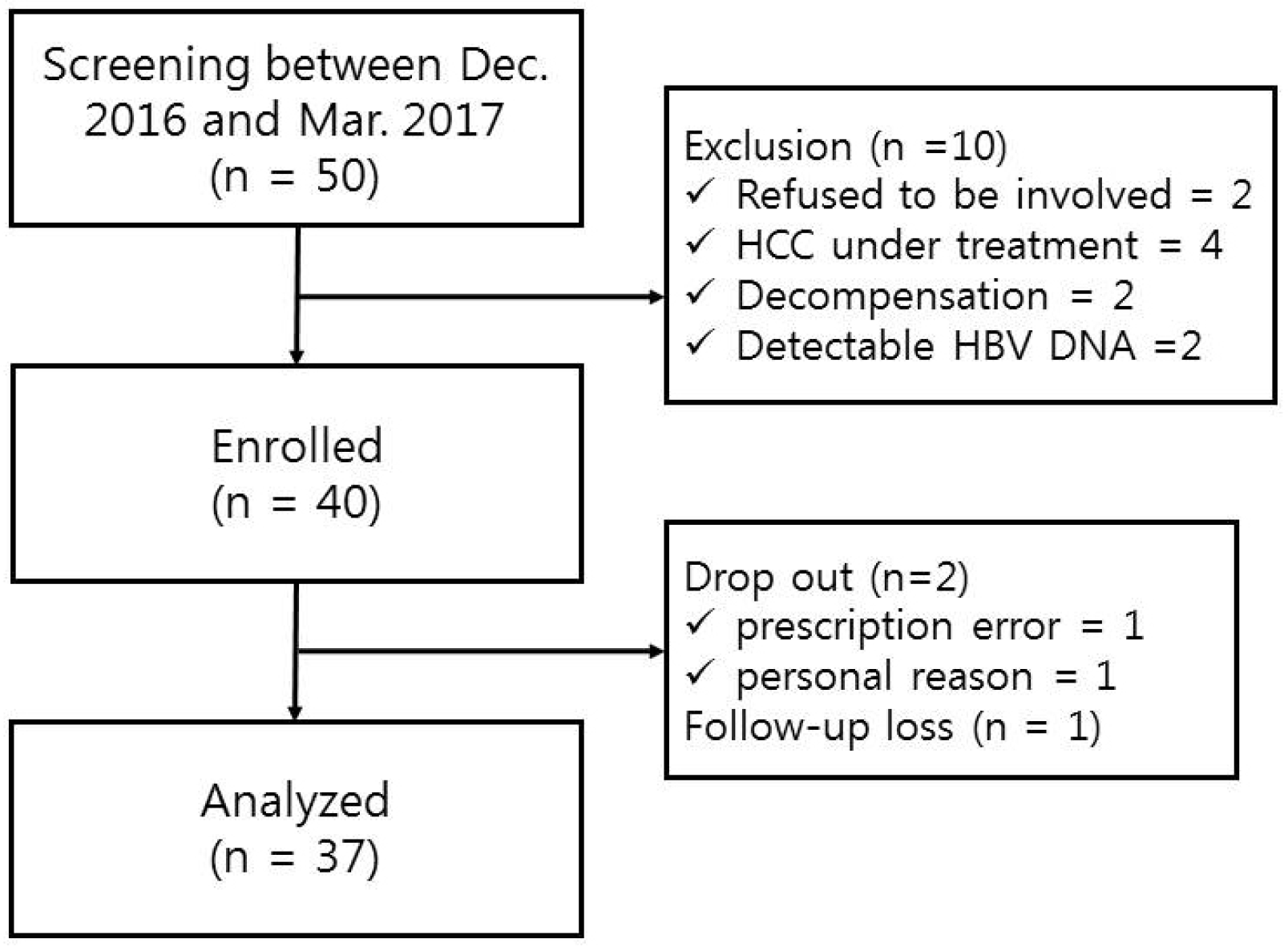
This study was a prospective single-arm open-label trial at the Department of Internal Medicine, Korea University Medical Center, South Korea, from December 2016 to March 2019. The primary endpoint was undetectable HBV DNA (<20 IU/mL) at 12 months after switching treatment. The secondary endpoints were the biochemical and serologic responses, viral breakthrough, antiviral resistance, and clinical adverse events at 12 months after switching treatment.
CHB patients receiving treatment with Baraclude® 1 mg alone or in combination with other nucleos(t)ide analogs for 12 months or longer after the development of antiviral resistance were screened. All subjects had controlled viral replication (HBV DNA <20 IU/mL at two or more time points) and agreed to participate in the present study by providing written informed consent.
Patients who satisfied the inclusion and exclusion criteria switched from Baraclude® 1 mg to Baracle®. Every 3 months, after the start of the clinical trial, scheduled tests were performed, which include biochemistry, hepatitis B e antigen (HBeAg), anti-HBe, and HBV DNA tests. An assessment of the treatment response at 12 months was performed by comparing the undetectable HBV DNA rates between the baseline and 12 months after switching therapy.
3. Patients
This study planned to analyze 34 or more subjects by enrolling 40 patients who satisfied the inclusion and exclusion criteria. The number of patients needed was calculated and is described in the statistical analyses section.
The inclusion criteria were as follows: 1) age >19 years old and diagnosis of CHB by positive hepatitis B surface antigen (HBsAg) for more than 6 months, and confirmed antiviral resistance for nucleos(t)ide analogs once or more in the past; 2) taking entecavir 1 mg alone or in combination with other drugs (adefovir, tenofovir, etc.) for more than 1 year; 3) HBV DNA <20 IU/mL measured at two or more time points at three-month intervals; 4) compensated liver cirrhosis (Child-Pugh-Turcotte score ≤7, prothrombin time ≤3 seconds above the upper limit of normal or international normalized ratio ≤1.5, serum albumin >3 g/dL, total bilirubin <2.5 mg/dL, no history of variceal bleeding, ascites requiring diuretic administration, or paracentesis and hepatic encephalopathy; and 5) understanding the need and process of clinical trials and agreed in writing to participate.
The exclusion criteria were as follows: 1) failure to meet the inclusion criteria; 2) serum creatinine level ≥1.5 mg/dL; 3) hepatitis C antibody positivity; 4) decompensated cirrhosis;
5) currently pregnant or lactating; 6) need for continuous treatment for hepatocellular carcinoma (HCC) or other untreated malignant tumors; or 7) regular consumption of a significant amount of alcohol (males, ≥140 g/week; females, ≥ 70 g/week).
4. Assays
Assays were performed (HBV DNA quantification, HBsAg, HBeAg, and anti-HBe tests) at the Department of Laboratory Medicine, Korea University Ansan Hospital. The lower detection limit of HBV DNA quantification was <20 IU/mL by a real-time polymerase chain reaction using COBAS AmpliPrep-COBAS TaqMan HBV test™, v2.0 (Roche Diagnostics, Branchburg, NJ, USA).
5. Criteria for dropout
Patients were removed from the study when medication compliance was not maintained (failure to take medication for more than 28 days during the 12 months), if the visiting schedule was violated twice or more during the study period, if they took nucleos(t)ide analogs other than those prescribed in this trial, or if any serious adverse events occurred.
6. Statistical analysis
The primary endpoint was undetectable HBV DNA at 12 months after switching treatment. Based on previous studies, the rate of undetectable HBV DNA at 12 months after enrollment was assumed to be 97.8%.
16 The following assumptions were made to confirm that the rate of undetectable HBV DNA at 12 months after switching therapy (test drug, or Baracle
®) was not inferior to the rate of undetectable HBV at the baseline when therapy was not changed (control drug or Baraclude
®). The number of subjects required in the non-inferiority test was calculated using the SAS 6.4 (SAS Institute, Cary, NC, USA) program with a 0.025 significance level (α), 0.8 statistical power (1-β), and 10% limit of clinical non-inferiority (ε) according to the following formula:
The calculation determined that 34 patients would be needed. Forty subjects were required in this study, considering a dropout rate of 15%.
All statistical analyses were performed using SPSS 18.0 (SPSS, Chicago, IL, USA), and p-values <0.05 were considered significant. The t-test was used for the continuous variables, while a chi-square test was used for the categorical variables.
For safety analysis, the incidence of clinical or laboratory abnormalities was calculated for each treatment group. Adverse events related to safety issues were assessed using the Common Terminology Criteria for Adverse Events v5.0 (
https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm). A Fisher’s exact test or chi-square test was used to assess the association between the adverse events and the test drug.
Go to :

DISCUSSION
Access to treatment is an important factor for achieving the World Health Organization goal of eliminating HBV infection by 2030.
17 The increased availability of generic antivirals has likely improved access to treatment.
18 Several international guidelines recommend newer agents, such as entecavir and tenofovir as a first-line treatment because they have high barriers to resistance.
3,7 Thus, the accessibility of generic entecavir and tenofovir will play an important role in controlling HBV infections worldwide.
The median price of generic tenofovir on the international market decreased from $208/person-year in 2004 to $38/person-year in 2014.
19 In contrast, the lowest reported price of entecavir was $427/person-year in 2015.
20 This means that generic entecavir must become less expensive, considering the current price of generic tenofovir, through the development of more generic products.
A major concern regarding generic agents is whether they show comparable efficacy and safety to the brand name drugs. In a survey, 25% of physicians said they would prescribe generic drugs more often if additional clinical trials were offered. Therefore, this study could provide important evidence for the selection of generic entecavir by physicians.
18
In addition, the daily dosage of entecavir is very low (0.5 or 1 mg), which means that the requirement for the active pharmaceutical ingredient is low, resulting in a low manufacturing cost. Some reports estimate the minimum target prices for entecavir at $36/person-year, which is substantially lower than the current generic prices and below those of tenofovir.
20
Therefore, encouraging the widespread competitive generic production of entecavir would enable dramatic price reductions and rapid scale-up of HBV treatments globally. To this end, a study on the efficacy and safety of generic entecavir and recommendations for its use will be needed.
In this regard, a randomized controlled trial comparing a new generic drug, Baracle
® 0.5 mg (Dong-A ST), and the original drug, Baraclude
® 0.5 mg, was recently conducted in treatment-naïve CHB patients.
21 On the other hand, an evaluation of generic entecavir 1 mg, which is indicated for antiviral-resistant HBV, has not been performed.
This study evaluated the safety and efficacy of switching to the entecavir 1 mg of a generic drug in CHB patients taking brand-name entecavir 1 mg as a mono-or combination therapy after the development of antiviral resistance to previous nucleos(t)ide analogs.
In this single-arm study, no significant difference in the viral detection rate was noted between before and after changing the drug. No inferiority was noted in the maintenance of undetectable HBV DNA after switching therapy compared to the baseline. As a result, no difference in the clinical effect was observed between the standard and test drugs. Similarly, no difference in biochemical response was observed after changing the drug. Serum HBeAg loss was confirmed in 12.5% of patients, but the clinical significance was limited. No major adverse effects were reported, and the incidence of recurred or newly developed HCC was considered unrelated to the drug; recurred or newly developed HCC was observed in the ETV+TDF group, of which the patients were treated more heavily and had more advanced liver diseases judged by the lower platelet counts. Overall, these findings suggest that generic entecavir, Baracle® 1 mg, is comparable to brand-name entecavir, Baraclude® 1 mg.
This study had several limitations to the present study. First, it was not a randomized control trial, which is an ideal study design for comparing two drugs. On the other hand, although it was a single-arm study, it demonstrated non-inferiority of the virologic response rate between the last time brand-name entecavir 1 mg was prescribed and after 12 months of generic entecavir 1 mg treatment.
Second, there was a limitation in evaluating the effectiveness of generic entecavir alone. As the study included patients taking entecavir 1 mg due to the antiviral resistance to previous nucleos(t)ide analogs, this study should have also included patients who were being prescribed this drug combined with other drugs. On the other hand, the generic entecavir mono-therapy group did not show any evidence of HBV DNA elevation during the 12 months of treatment, although the cohort size was insufficient. Switching to entecavir 1 mg monotherapy from adefovir-based combination therapies might be considered in limited situations,
22,23 but it would not be effective switching from the tenofovir-based combination therapy.
15 Hence, combination therapy needs to be ensured in multiple treatment failures.
15 Third, the kinetics of the decline in viral replication was not compared because all the patients already had undetectable HBV DNA at enrollment. Nevertheless, a study design comparing the rates of maintaining undetectable HBV DNA would be relevant because the incidence of newly developed antiviral resistance has decreased after introducing high genetic barrier drugs.
1,24,25
Entecavir 1 mg is an important medication prescribed frequently either alone or in combination in a variety of situations involving resistance to antiviral agents. A previous study compared the efficacy of generic entecavir 0.5 mg with the brand name drug for treatment naïve CHB. To the best of the authors’ knowledge, this study is the first to evaluate the clinical efficacy and safety of generic entecavir 1 mg. Therefore, this study provides important evidence for selecting generic drugs in cases of antiviral resistance.
In conclusion, generic entecavir (Baracle®) 1 mg, is a reasonable alternative to brand-name entecavir 1 mg, in CHB patients with compensated liver diseases who are resistant to previous nucelos(t)ide analogs with viral suppression.
Go to :





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download