Abstract
Background/Aims
Escherichia coli Nissle 1917 (EcN) alone therapy is as effective as mesalamine in inducing and maintaining remission in ulcerative colitis (UC). The efficacy and safety of EcN in combination with standard therapies have not been studied. This study examined the changes in the inflammation markers and symptoms following the additional administration of EcN to patients showing the clinical remission of UC.
Methods
UC patients who received EcN after being in clinical remission for more than 3 months at Kosin University Gospel Hospital between 2013 and 2018 were evaluated through the retrospective medical-record-based review. The partial Mayo score, fecal calprotectin (FC), BMI, hemoglobin, serum cholesterol, serum albumin levels, and the safety profiles were examined at 3rd and 6th months after initiating EcN.
Results
Ninety-four patients were included. After 3 months of treatment, there was no significant change in FC (156.3 μg/g to 141.1 μg/g) (p=0.653). On the other hand, partial Mayo score decreased significantly from 0.085 to 0.014 (p=0.025), and the bodyweight (p=0.001), BMI (p<0.001), hemoglobin (p=0.009), and cholesterol level increased (p=0.148). One patient (1.1%) experienced a serious adverse event with UC flare-up, and 14 patients (14.9%) discontinued EcN due to adverse events; all developed within 3 months.
Ulcerative colitis (UC) can be treated with 5-aminosalicylic acid (5-ASA), thiopurine, and biologics as the standard therapies.1 These medications can be added or removed according to the patients’ specific symptoms and inflammation status. The current treatment goal of inflammatory bowel disease (IBD) is to prevent the surgery and complications that could lead to disability.2 To change the natural course of IBD, reducing intestinal inflammation before symptom manifestation is important. The treatment priority has shifted from symptom control to the reduction of inflammation.3-5 Nevertheless, in many cases, inflammation is not controlled sufficiently and high levels of inflammation persist even in symptomatic remission status.
IBD is characterized by an imbalance of intestinal microbiota, and the depletion of gut microbiota causes defects in mucosal healing and chronic mucosal inflammation.6 Strategies to manipulate the microbiome such as diet, probiotics, antibiotics, and fecal microbiota transplantation can be used therapeutically to modulate the activity of IBD.7 Probiotics are non-pathogenic, living microbial populations that can increase beneficial microorganisms in the intestine and have a positive effect on the balance of intestinal microbiota. This may have a long-term effect on reducing inflammation during the course of IBD.8 Among these probiotics, previous studies have shown that Escherichia coli Nissle 1917 (EcN; Mutaflor, Ardeypharm GmbH, Herdecke, Germany) is comparable to 5-ASA for the induction and maintenance of remission in patients with UC.9-14 EcN is a non-pathogenic, Gram-negative bacterium belonging to the Enterobacteriaceae family and helps maintain intestinal homeostasis.15 In contrast to other E. coli strains, EcN does not produce virulence factors and intestinal mucosal damage.
Previous studies of EcN included a small number of UC patients and only compared EcN with a low dose mesalazine single treatment.16 Although there has been an increasing demand for knowledge of the combination therapy of EcN with standard UC treatments, no study has examined them.17
This study examined the changes in UC symptoms, inflammation markers, and serologic tests after administering EcN to clinically remission-attained UC patients at a tertiary IBD care center in real-world clinical practice.
This investigation was an uncontrolled, observational, retrospective study using a hospital-based UC cohort. The medical records of all patients who took EcN and were in a UC steroid-free remission state for at least 3 months at Kosin IBD clinics from December 1, 2013, to November 14, 2018, were reviewed. The definition of UC clinical remission was a partial Mayo score (pMS) of 0-1.18 Patients with any malignancy, previously taking other probiotics, and active disease status were excluded. All patients in remission had routinely visited every 3 months to evaluate the BMI and pMS, and blood tests including a complete blood count, liver chemistry, blood lipid profile, and FC test. The study protocol was approved by the Institutional Review Board of the Kosin University Gospel Hospital (IRB No. 2018-11-008).
EcN was taken as one capsule twice daily (2.5×109 viable bacteria per capsule) and required refrigeration (temperature between +2℃ and +8℃) for storage. The patients’ current UC standard medications included mesalamine 4 g daily, azathioprine (adjusting dose according to the patient’s symptoms, mean 1.01 mg/kg, range 0.22-2 mg/kg), mesalamine 1 g enema daily, and mesalamine 1 g suppository daily. During EcN administration, the standard therapies were not changed except in cases of UC flare-up.
The changes in FC levels for the intestinal inflammation, pMS, body weight, BMI, hemoglobin (Hb), serum total cholesterol, and albumin levels were examined at the third month and the sixth month following the administration of EcN to UC patients. The safety profile of EcN was reviewed to determine the reasons for EcN discontinuation. The reasons of discontinuations were divided into symptoms change (doctor’s suggestion) or individual issues (patient request). The discontinuations by the doctor’s suggestion were classified into three groups: possible, unlikely, and no association with EcN. The survival rate of EcN discontinuation was analyzed during the one-year observation period.
The FC, pMS, body weight, BMI, Hb, albumin, and total cholesterol of each patient were compared over time using a Wilcoxon signed-rank test. This was not to compare the mean value of the entire group but to compare the individual patients’ changes after taking EcN. The analysis was conducted on patients who completed the administration of EcN until the next outpatient visit. If the medication was not taken at the evaluation time point or if blood or stool samples were not obtained, the missing values were excluded from statistical analysis. The statistical significance was based on the 95% CI (p<0.05). The Kaplan-Meier curve was drawn by examining the number of patients who discontinued the EcN treatment during the 1-year observation period. The Statistical Package for Social Science (SPSS 21, IBM Corp, Armonk, NY, USA) was used for statistical analysis.
Ninety-four patients were enrolled in this study. The demographics and clinical baseline characteristics of the patients were as follows (Table 1): 76, 68, and 62 patients underwent EcN treatment for 3 months, 6 months, and 1 year, respectively. The majority took 5-ASA (n=85, 90.4%) and 29 patients (30.9%) took thiopurine. None of the patients were taking biologics, and two patients (2.1%) did not take any standard medication due to the adverse effects of both 5-ASA and thiopurine before EcN medication initiation.
Fig. 1 presents the changes in FC, in which the image on the left shows the changes after 3 months in 60 patients, and the image on the right shows the changes for 6 months in 50 patients. Initially, the FC level was 156.3 μg/g and decreased to 141.1 μg/g at the 3rd month. The number of patients whose FC decreased was 27 (p=0.653). Fig. 2 shows the number of patients who demonstrated an FC change >50 μg/g between each time point. Ten patients demonstrated a decrease at the 3rd month after initiating EcN treatment. Thirteen patients demonstrated an increase at the 3rd month after initiating treatment.
In previous meta-analyses, various cut-off values of FC were suggested to predict the flare-up of the disease.19,20 Among the upper cut-off values suggested, 50 μg/g was the lowest, and the average value was approximately 150 μg/g. The number of patients whose FC level was greater than 50 μg/g was initially 20 and increased to 23 patients at 3 months. The initial FC level of 12 patients was >150 μg/g at both the start and the 3rd month. In the group of patients who underwent EcN treatment for 6 months, the number of patients whose FC level was greater than 50 μg/g was initially 19 and increased to 21 patients at 6 months.
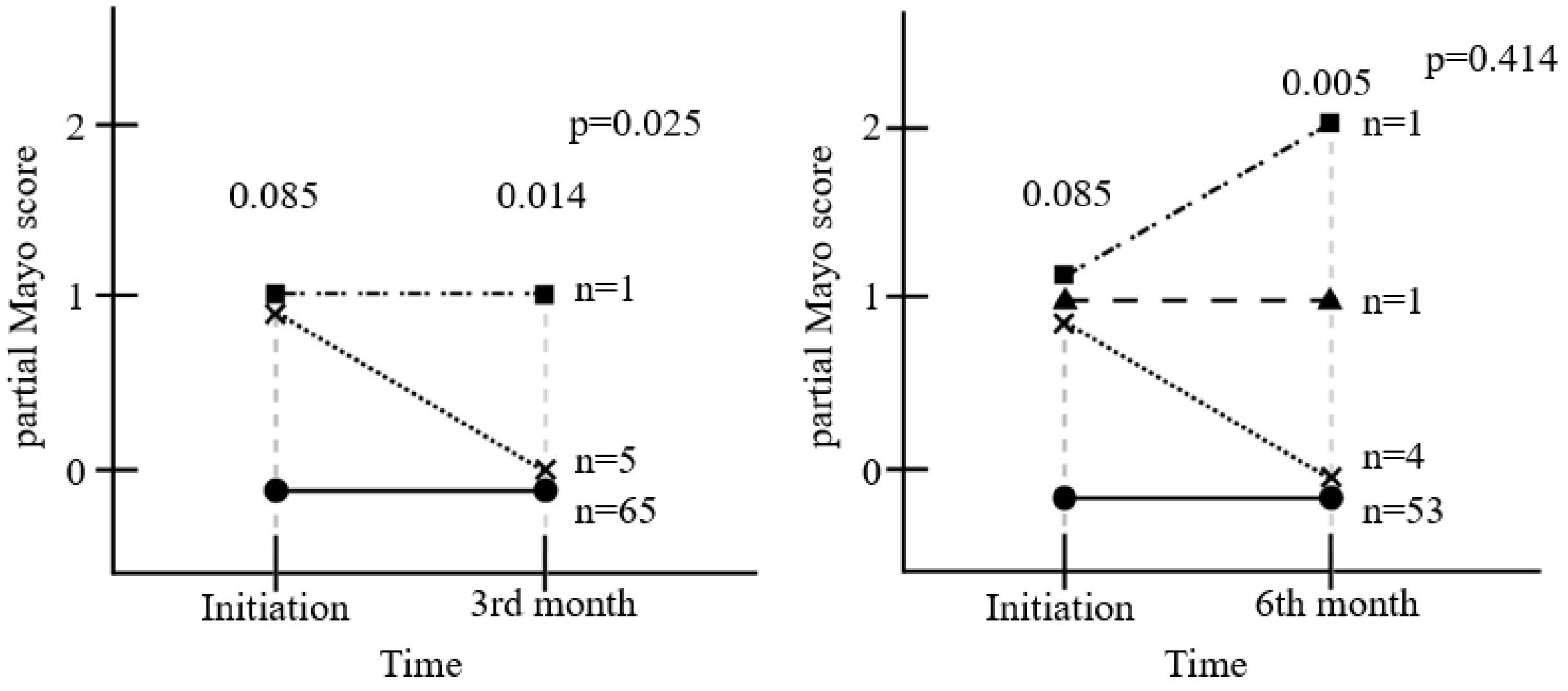
The pMS was assessed in 71 patients at 3 months (Fig. 3). Initially, six patients had one point, and 65 patients had zero points. The pMS of five in 71 patients decreased from one to zero points (p=0.025). During the 6 months of treatment, six patients had one point and 53 patients had zero points at the baseline. After 6 months, four of the six patients decreased to zero points, and one patient increased to two points; one patient had one point, and 53 patients had zero points at 6th month (p=0.414).
Seven patients stopped EcN due to a worsening of symptoms in the early 3 months, and their pMS at their 3rd-month outpatient visit was 4.25 points (range 3-8). Even including the seven patients who stopped the medication at 3rd month and they had high pMS score, the pMS had decreased (p=0.031).
Fig. 4 shows the changes in body weight, BMI, Hb, and albumin. At the 3rd month after initiating EcN treatment, the bodyweight increased from 66.05 kg to 66.62 kg (p=0.001); the BMI increased from 23.75 kg/m2 to 23.99 kg/m2 (p<0.001); the Hb increased from 13.95 g/dL to 14.15 g/dL (p=0.009), and the albumin level decreased from 4.64 g/dL to 4.55 g/dL within the normal range (p=0.008). Furthermore, the serum total cholesterol increased from 185 mg/dL to 190 mg/dL (p=0.148). A similar trend was observed at 6 months.
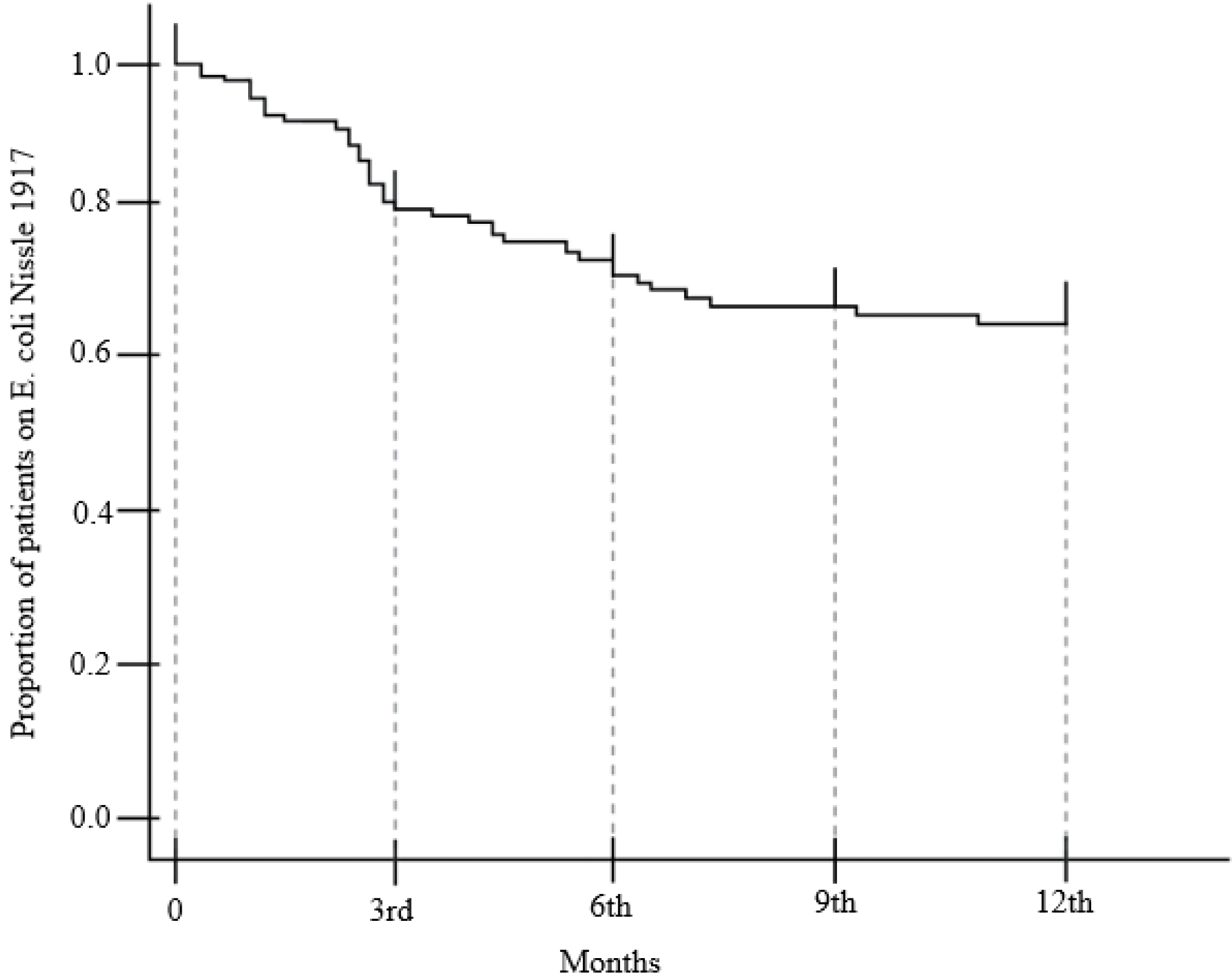
Sixty-two of the 94 patients showed compliance with the EcN doses for one year (65.96%). Fig. 5 presents the Kaplan-Meier survival curve, according to the dosing period of 32 discontinued patients. Fig. 6 outlines the reasons for EcN discontinuation (n=32), which were divided into the doctor’s suggestion (n=20) or the patient’s request (n=12).
The discontinuations related to the doctor’s opinion were classified into the following: possible (n=7), unlikely (n=7), and no (n=6) association with EcN. 1) Seven patients presented with a pMS >5, were considered to be possibly associated with EcN treatment, and required the discontinuation of EcN and the initiation of steroid therapy. One of the seven patient presented with hematochezia and diarrhea on day 7th after initiating the EcN treatment requiring hospitalization with intravenous steroid therapy. 2) Seven patients presented with abdominal symptoms that were not severe enough to consider UC flare-up. For these patients, the symptoms were considered unlikely to be associated with the EcN treatment, but it was discontinued, and no steroid treatment was needed. 3) The symptomatic events in the remaining six patients were considered to be not associated with the EcN treatments.
Most of 12 patients discontinued EcN due to the patient’s request without adverse events. One patient was lost to follow-up. The other eleven patients discontinued due to the following reasons: the cost burden of EcN (n=6), reluctance to take many drugs in addition to existing medications (n=3), and inconvenience in keeping EcN refrigerated (n=2).
This study was an uncontrolled, observational, retrospective study to examine the changes in inflammation markers and symptoms following administration of EcN to clinical-remission UC patients in real-world clinical practice. The FC, pMS, weight, BMI, Hb, albumin, and serum total cholesterol were checked to determine the change in intestinal inflammation and symptoms after the initiation of EcN. Initially, 94 patients were treated, 76 patients had been on medication for 3 months, and 68 patients had been on medication for 6 months.
The level of mean FC numerically decreased from 156.3 μg/g to 141.1 μg/g during the first 3 months of administration, but the change for each patient was not statistically significant (p=0.653). No relationship between the FC change and EcN treatment was observed in this analysis.
Although there have been previous meta-analyses that reported a positive association between FC and acute exacerbation of UC, there is no clear consensus regarding the degree of FC increase to confirm the changes in intestinal inflammation.19,20 Although the cut-off value of FC among these previous meta-analyses studies varied, the minimal cut-off value was approximately 50 μg/g. Therefore, this study considered the potential change in intestinal inflammation was the change in FC >50 μg/g. In the present study, only 10 patients showed a decrease in FC >50 μg/g after 3 months of EcN treatment, whereas an increase in FC >50 μg/g was observed in 13 patients. The FC of 20 out of 60 patients was >50 μg/g, while the FC of 12 patients was >150 μg/g at the baseline. After the first 3 months, FC was >50 μg/g in 23 patients, and FC was >150 μg/g in 12 patients. Taking EcN does not decrease the FC significantly and regulate intestinal inflammation, even though the mean FC level decreased at 3 months.
In previous studies examining the effects of EcN on the induction and maintenance of remission of UC, the disease status was assessed using the clinical activity index (CAI) or the Truelove and Witt score.9,10,13,21 The pMS was used for assessment in this present study. The pMS consists of the stool frequency, rectal bleeding, and physician's global assessment. Six out of 71 patients had mild UC symptoms with a pMS of one point. Among the six patients, five showed improved symptoms, decreasing the pMS to 0 points at the 3rd month (p=0.025). Of the five patients with a decrease, four patients showed a decreased stool frequency, and one patient was in the physician’s global assessment. EcN may have some benefit for bowel symptoms and life quality in UC patients.
Currently, the reduction of intestinal inflammation is considered as an important target for disease control, and the degree of inflammation can be determined by the level of FC.14 Although this study hypothesized that EcN treatment would reduce inflammation and improve symptoms, the results indicate that there were no notable changes in intestinal inflammation as the result of administrating EcN, but only the improvement of symptoms. The administration of EcN resulted in improving UC symptoms in only 3 months. The intestinal flora can change very quickly and is sensitive to even minor changes, including antibiotic administration.22 In the group given the 6-month treatment duration, the pMS did not improve compared to the initial pMS (p=0.414). Thus, pMS after administering EcN showed improvement within 3 months, which was maintained for the next 3 months; this improvement was inconsistent.
The body weight and BMI increased gradually after initiating the EcN treatment. The body weight increased significantly after 3 months compared to the baseline values (p=0.001). The BMI also increased at 3 months (p<0.001). Hb increased at 3 months compared to the baseline values (p=0.009). Compared to normal subjects, UC patients experienced malnutrition due to persistent intestinal inflammatory conditions and weight loss.23,24 Previous studies on the nutritional status of patients with IBD showed that weight, BMI, Hb, ferritin, albumin, and total cholesterol levels were reduced significantly which could be used as an index to evaluate the malnutrition status in these patients. In this study, patients who received EcN as a combination therapy showed a gradual increase in weight, BMI, Hb, and total cholesterol which are general factors associated with the nutritional status. This suggests that EcN may help improve the patient’s nutritional status. In addition, as the weight was gained during the observation period, some patients showed active weight control through diet and exercise which might helped improve the patients' overall activity. It has been speculated that stabilization of the intestinal barrier due to EcN can decrease nutritional loss, thereby increasing weight gain.25,26 Weight gain is important for patients with UC to improve the quality of life, but weight loss in patients with IBD increases the risk of fractures and compromises immunity.27 Unlike other factors, it is unique that albumin decreases. Albumin is not only a result of the amino acid metabolism; it is also an acute-phase protein that is consumed under inflammatory conditions.28 For this reason, although EcN was taken, the intestinal inflammation was not reduced sufficiently, so the albumin level could be reduced. Follow-up studies will be needed to clarify these hypotheses.
This study analyzed the adverse events that occurred during the observation period. In these cases, however, the causal relationship between taking EcN and symptomatic aggravation is unclear. Fig. 6 presents all records of EcN discontinuation. Most discontinuation due to gastrointestinal symptoms occurred less than 3 months after initiating the EcN treatment. Therefore, if clinicians are concerned about the side effects of EcN, they should observe the patients for the first 3 months after EcN administration. There were no unexpected or novel side effects of EcN in this study. This is a similar result to a previous study that EcN was relatively safe.9,10,12,13,15 Among the patients taking EcN, some discontinued treatment due to cost issues, reluctance to take many drugs, and inconvenience in keeping EcN refrigerated. To improve the compliance of EcN treatment and increase the potential benefits, clinicians will need to provide repeated explanations and education to their patients.
In previous studies, the dosage of 5-ASA was relatively low, and the population sizes were small. Therefore, these previous studies did not fully address the questions posed by the current IBD clinicians. As a result, the administration of EcN alone was rare in clinical settings, except in situations involving the side effects of 5-ASA and thiopurine.29-31 Unlike previous studies, this study used EcN combined with the current standard treatments for UC. In this study, the standard UC drug that 85 patients took to maintain their remission was 5-ASA. On the other hand, seven patients took thiopurine alone due to the side effects of 5-ASA such as pancreatitis, nausea, and allergic reaction. Two patients took neither 5-ASA nor thiopurine due to the side effects of pancreatitis caused by 5-ASA as well as hepatitis and allergic reactions caused by thiopurine. These two patients underwent EcN treatment for one year without experiencing any specific adverse events; EcN may be an additional alternative treatment for maintaining UC remission to patients who experienced side effects with the standard UC treatments.
The main limitation of this study was the single-center, uncontrolled study design. UC does not have a single mechanism and is influenced by genetic, geographic, and environmental factors.32 Therefore, a multi-center study with controls is needed to clarify the effects of EcN further. On the other hand, an attempt was made to overcome these limitations by collecting data from a homogeneous population who maintained remission for at least 3 months and did not change the underlying UC treatment regimen and dosing of medication from at least 3 months before and during the entire EcN treatment. As this study only described body weight and BMI, future studies could investigate the changes in body composition and muscle proportion in the body.33 FC was analyzed as a biological marker associated with intestinal inflammation for the treatment of UC, but there was no significant difference after the EcN treatment. The endoscopic findings can indicate inflammation status and help determine the effects of EcN; further studies will be needed to investigate the endoscopic findings with EcN therapy.
In conclusion, the administration of EcN to clinically remission-attained UC patients may further improve symptoms without changing the intestinal inflammation markers. Most EcN-associated adverse events develop within the early few weeks.
ACKNOWLEDGEMENT
Dr. Won Moon has received research funding from BL&H Co., Ltd. The other authors have nothing to declare.
REFERENCES
1. Harbord M, Eliakim R, Bettenworth D, et al. 2017; Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J Crohns Colitis. 11:769–784. DOI: 10.1093/ecco-jcc/jjx009. PMID: 28513805.


2. Panes J, O'Connor M, Peyrin-Biroulet L, Irving P, Petersson J, Colombel JF. 2014; Improving quality of care in inflammatory bowel disease: what changes can be made today? J Crohns Colitis. 8:919–926. DOI: 10.1016/j.crohns.2014.02.022. PMID: 24713174.


3. Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD. 2019; ACG clinical guideline: ulcerative colitis in adults. Am J Gastro- enterol. 114:384–413. DOI: 10.14309/ajg.0000000000000152. PMID: 30840605.

4. Choi CH, Moon W, Kim YS, et al. 2017; Second Korean guideline for the management of ulcerative colitis. Korean J Gastroenterol. 69:1–28. DOI: 10.4166/kjg.2017.69.1.1. PMID: 28135789.


5. Torres J, Burisch J, Riddle M, Dubinsky M, Colombel JF. 2016; Preclinical disease and preventive strategies in IBD: perspectives, challenges and opportunities. Gut. 65:1061–1069. DOI: 10.1136/gutjnl-2016-311785. PMID: 27196600.


6. Morgan XC, Tickle TL, Sokol H, et al. 2012; Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 13:R79. DOI: 10.1186/gb-2012-13-9-r79. PMID: 23013615. PMCID: PMC3506950.

7. Lane ER, Zisman TL, Suskind DL. 2017; The microbiota in inflammatory bowel disease: current and therapeutic insights. J Inflamm Res. 10:63–73. DOI: 10.2147/JIR.S116088. PMID: 28652796. PMCID: PMC5473501.



8. Frank DN, Robertson CE, Hamm CM, et al. 2011; Disease phenotype and genotype are associated with shifts in intestinal-associated microbiota in inflammatory bowel diseases. Inflamm Bowel Dis. 17:179–184. DOI: 10.1002/ibd.21339. PMID: 20839241. PMCID: PMC3834564.


9. Matthes H, Krummenerl T, Giensch M, Wolff C, Schulze J. 2010; Clinical trial: probiotic treatment of acute distal ulcerative colitis with rectally administered Escherichia coli Nissle 1917 (EcN). BMC Complement Altern Med. 10:13. DOI: 10.1186/1472-6882-10-13. PMID: 20398311. PMCID: PMC2861635.



10. Kruis W, Schutz E, Fric P, Fixa B, Judmaier G, Stolte M. 1997; Double-blind comparison of an oral Escherichia coli preparation and mesalazine in maintaining remission of ulcerative colitis. Aliment Pharmacol Ther. 11:853–858. DOI: 10.1046/j.1365-2036.1997.00225.x. PMID: 9354192.

11. Losurdo G, Iannone A, Contaldo A, Ierardi E, Di Leo A, Principi M. 2015; Escherichia coli Nissle 1917 in Ulcerative Colitis Treatment: Systematic Review and Meta-analysis. J Gastrointestin Liver Dis. 24:499–505. DOI: 10.15403/jgld.2014.1121.244.ecn. PMID: 26697577.
12. Kruis W, Fric P, Pokrotnieks J, et al. 2004; Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut. 53:1617–1623. DOI: 10.1136/gut.2003.037747. PMID: 15479682. PMCID: PMC1774300.


13. Rembacken BJ, Snelling AM, Hawkey PM, Chalmers DM, Axon ATR. 1999; Non-pathogenic Escherichia coli versus mesalazine for the treatment of ulcerative colitis: a randomised trial. The Lancet. 354:635–639. DOI: 10.1016/S0140-6736(98)06343-0. PMID: 10466665.
14. Hajela N, Ramakrishna BS, Nair GB, Abraham P, Gopalan S, Ganguly NK. 2015; Gut microbiome, gut function, and probiotics: Implications for health. Indian J Gastroenterol. 34:93–107. DOI: 10.1007/s12664-015-0547-6. PMID: 25917520.


15. Scaldaferri F, Gerardi V, Mangiola F, et al. 2016; Role and mechanisms of action of Escherichia coli Nissle 1917 in the maintenance of remission in ulcerative colitis patients: An update. World J Gastroenterol. 22:5505–5511. DOI: 10.3748/wjg.v22.i24.5505. PMID: 27350728. PMCID: PMC4917610.


16. Sheehan D, Moran C, Shanahan F. 2015; The microbiota in inflammatory bowel disease. J Gastroenterol. 50:495–507. DOI: 10.1007/s00535-015-1064-1. PMID: 25808229.


17. Ganji-Arjenaki M, Rafieian-Kopaei M. 2018; Probiotics are a good choice in remission of inflammatory bowel diseases: A meta analysis and systematic review. J Cell Physiol. 233:2091–2103. DOI: 10.1002/jcp.25911. PMID: 28294322.


18. Lewis JD, Chuai S, Nessel L, Lichtenstein GR, Aberra FN, Ellenberg JH. 2008; Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm Bowel Dis. 14:1660–1666. DOI: 10.1002/ibd.20520. PMID: 18623174. PMCID: PMC2597552.



19. Mao R, Xiao YL, Gao X, et al. 2012; Fecal calprotectin in predicting relapse of inflammatory bowel diseases: a meta-analysis of prospective studies. Inflamm Bowel Dis. 18:1894–1899. DOI: 10.1002/ibd.22861. PMID: 22238138.


20. Maaser C, Sturm A, Vavricka SR, et al. 2019; ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. 13:144–164. DOI: 10.1093/ecco-jcc/jjy113. PMID: 30137275.


21. Schoepfer AM, Beglinger C, Straumann A, Trummler M, Renzulli P, Seibold F. 2009; Ulcerative colitis: correlation of the Rachmilewitz endoscopic activity index with fecal calprotectin, clinical activity, C-reactive protein, and blood leukocytes. Inflamm Bowel Dis. 15:1851–1858. DOI: 10.1002/ibd.20986. PMID: 19462421.

22. Gao K, Pi Y, Peng Y, Mu CL, Zhu WY. 2018; Time-course responses of ileal and fecal microbiota and metabolite profiles to antibiotics in cannulated pigs. Appl Microbiol Biotechnol. 102:2289–2299. DOI: 10.1007/s00253-018-8774-2. PMID: 29362824.


23. Geerling BJ, Badart-Smook A, Stockbrugger RW, Brummer RJ. 2000; Comprehensive nutritional status in recently diagnosed patients with inflammatory bowel disease compared with population controls. Eur J Clin Nutr. 54:514–521. DOI: 10.1038/sj.ejcn.1601049. PMID: 10878655.


24. Mijac DD, Jankovic GL, Jorga J, Krstic MN. 2010; Nutritional status in patients with active inflammatory bowel disease: prevalence of malnutrition and methods for routine nutritional assessment. Eur J Intern Med. 21:315–319. DOI: 10.1016/j.ejim.2010.04.012. PMID: 20603043.

25. Chaudhari AS, Raghuvanshi R, Kumar GN. 2017; Genetically engineered Escherichia coli Nissle 1917 synbiotic counters fructose-induced metabolic syndrome and iron deficiency. Appl Microbiol Biotechnol. 101:4713–4723. DOI: 10.1007/s00253-017-8207-7. PMID: 28283693.

26. Ukena SN, Singh A, Dringenberg U, et al. 2007; Probiotic Escherichia coli Nissle 1917 inhibits leaky gut by enhancing mucosal integrity. PLoS One. 2:e1308. DOI: 10.1371/journal.pone.0001308. PMID: 18074031. PMCID: PMC2110898.
27. Farraye FA, Melmed GY, Lichtenstein GR, Kane SV. 2017; ACG Clinical Guideline: Preventive Care in Inflammatory Bowel Disease. Am J Gastroenterol. 112:241–258. DOI: 10.1038/ajg.2016.537. PMID: 28071656.


28. Valentini L, Schulzke JD. 2011; Mundane, yet challenging: the assessment of malnutrition in inflammatory bowel disease. Eur J Intern Med. 22:13–15. DOI: 10.1016/j.ejim.2010.07.021. PMID: 21238886.


29. Di Paolo MC, Paoluzi OA, Pica R, et al. 2001; Sulphasalazine and 5-aminosalicylic acid in long-term treatment of ulcerative colitis: report on tolerance and side-effects. Dig Liver Dis. 33:563–569. DOI: 10.1016/S1590-8658(01)80108-0. PMID: 11816545.


30. Fiorentini MT, Fracchia M, Galatola G, Barlotta A, de la Pierre M. 1990; Acute pancreatitis during oral 5-aminosalicylic acid therapy. Dig Dis Sci. 35:1180–1182. DOI: 10.1007/BF01537594. PMID: 2390934.


31. de Jong DJ, Goullet M, Naber TH. 2004; Side effects of azathioprine in patients with Crohn's disease. Eur J Gastroenterol Hepatol. 16:207–212. DOI: 10.1097/00042737-200402000-00014. PMID: 15075996.


32. Sartor RB. 2006; Mechanisms of disease: pathogenesis of Crohn's disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol. 3:390–407. DOI: 10.1038/ncpgasthep0528. PMID: 16819502.


33. Valentini L, Schaper L, Buning C, et al. 2008; Malnutrition and impaired muscle strength in patients with Crohn's disease and ulcerative colitis in remission. Nutrition. 24:694–702. DOI: 10.1016/j.nut.2008.03.018. PMID: 18499398.


34. Silverberg MS, Satsangi J, Ahmad T, et al. 2005; Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol . 19 Suppl A:5A–36A. DOI: 10.1155/2005/269076. PMID: 16151544.

35. Ford AC, Bernstein CN, Khan KJ, et al. 2011; Glucocorticosteroid therapy in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol. 106:590–599. DOI: 10.1038/ajg.2011.70. PMID: 21407179.


Fig. 1
Fecal calprotectin over time: the thicker black line in the graph represents the mean value, and the gray lines represent the value for
each patient.
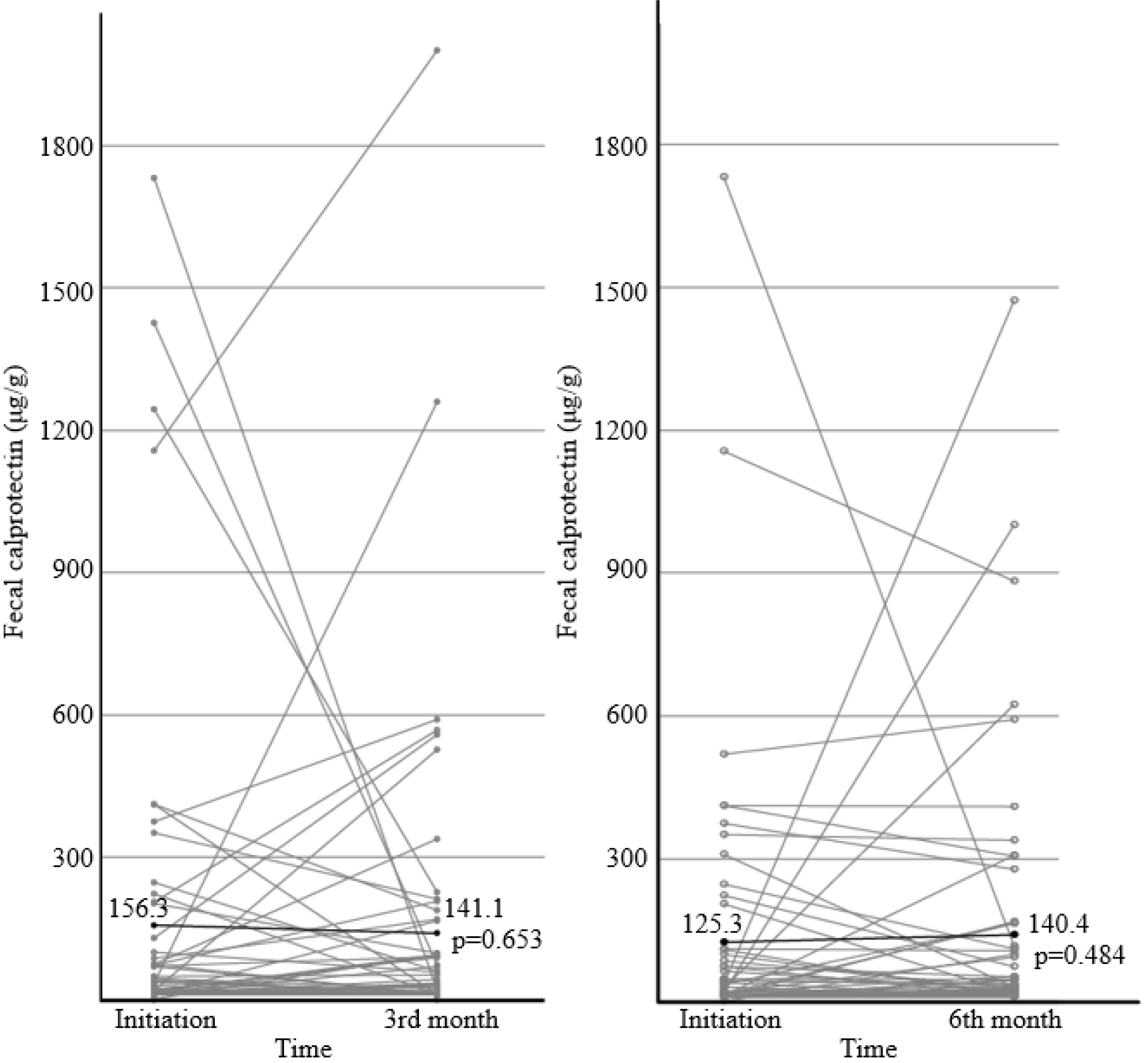
Fig. 2
Number of patients with fecal calprotectin level >50 μg/g or >150 μg/g, and the number of patients whose fecal calprotectin changed over 50 μg/g.
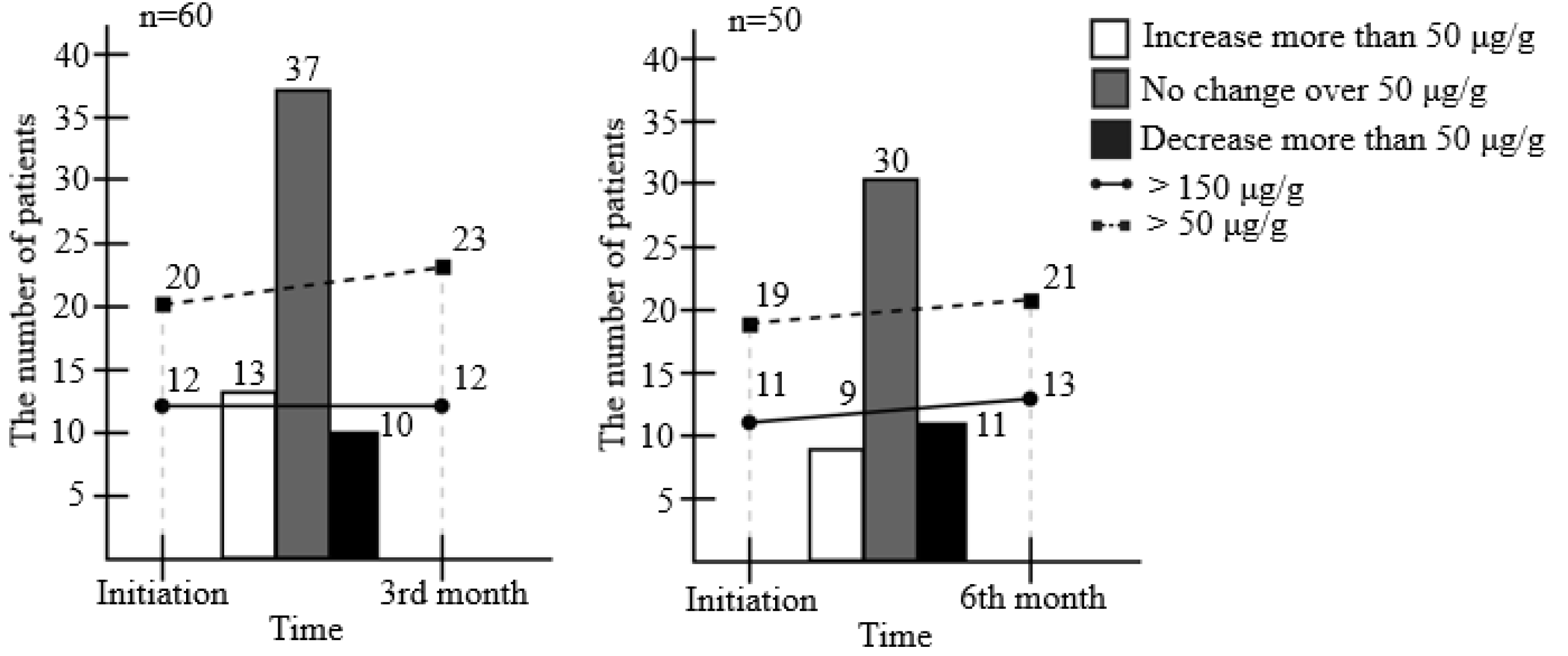
Fig. 6
Reasons for Escherichia coli Nissle 1917 (EcN) discontinuation, which were divided into (X, ▲, ●). All adverse events were classified into three, possible (X), unlikely (▲) association, and not (●) associated with EcN.
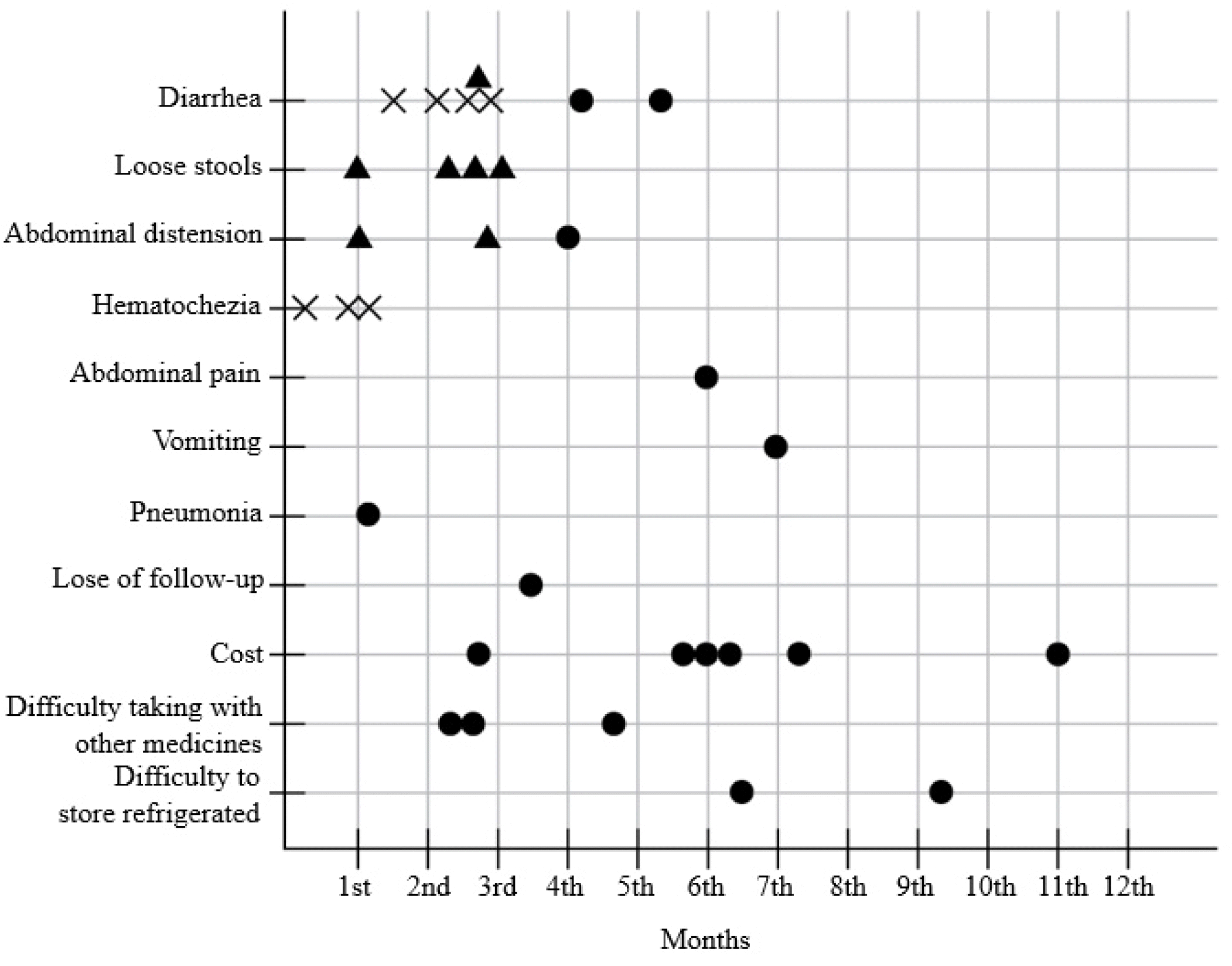
Table 1
Demographics and Clinical Characteristics of the 94 Patients Included in the Study
| Characteristics (n=94) | |
|---|---|
| Sex | |
| Male:female | 56 (59.6%):38 (40.4%) |
| Age (years) | 49.90 (23-76) |
| Duration after diagnosis (years) | 6.86 (1-20) |
| <3 | 21 (22.3%) |
| 3-6 | 28 (29.8%) |
| 7-9 | 27 (28.7%) |
| 10-12 | 9 (9.6%) |
| >12 | 9 (9.6%) |
| Disease extent according to Montreal classification34 | |
| Ulcerative proctitis (E1) | 27 (28.7%) |
| Left-sided ulcerative colitis (E2) | 30 (31.9%) |
| Extensive ulcerative colitis (E3) | 37 (39.4%) |
| Body weight (kg) | 65.75 (41-93) |
| BMI (kg/m²) | 23.70 (16.85-31.83) |
| <18.5 | 4 (4.3%) |
| 18.5≤ and <25 | 58 (61.7%) |
| 25≤ and <30 | 29 (30.9%) |
| 30≤ and <35 | 3 (3.2%) |
| ≥35 | 0 (0.0%) |
| History of previous steroid medicationa | 57(60.6%) |
| Concomitant medication | |
| 5-ASA, per-oral | 85 (90.4%) |
| 5-ASA, topical agent | 42 (44.7%) |
| Thiopurine | 29 (30.9%) |
| Biologics | 0 (0.0%) |
| None | 2 (2.1%) |
aCase where 40 mg or more of oral prednisolone had been administered for at least 1 week.35




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download