Abstract
Thrombocytopenia-associated multiple organ failure (TAMOF) is a distinct type of sepsis related to thrombocytopenia, microangiopathic hemolysis, and multiple organ failure. TAMOF belongs to a spectrum of syndromes related to disseminated intravascular coagulopathy, thrombotic thrombocytopenic purpura, and hemolytic uremic syndrome. Treatment modalities for TAMOF include therapeutic plasma exchange along with other extracorporeal organ support. To the best of our knowledge, we report the first case of triple-modality treatment for TAMOF in Korea, which involved extracorporeal membrane oxygenation, continuous renal replacement therapy, and therapeutic plasma exchange in a patient with TAMOF
초록
혈소판감소증을 동반한 다발성 장기부전은 패혈증이 진행되어 나타나는 중증 임상 상태로 파종혈관내응고, 혈전혈소판감소자색 반병, 그리고 용혈성요독증후군과 관련이 있다. 치료로는 혈장교 환술 및 여러 체외 생명유지 장치가 동시에 요구된다. 본 증례에서는 혈장교환술, 체외막산소화요법, 그리고 지속적 신대체요법을 동시에 진행하였기에 이를 보고한다.
During the progression of multiple organ failure, new-onset thrombocytopenia is regarded as a hazardous sign indicating that thrombocytopenia-associated multiple organ failure (TAMOF) may have developed [1, 2]. This condition may require the application of extracorporeal organ supporting approaches, including extracorporeal membrane oxygenation (ECMO), continuous renal replacement therapy (CRRT), and therapeutic plasma exchange (TPE). Simultaneous dual-modality therapy has been frequently performed to treat TAMOF around the world. However, simultaneous triple-modality therapy has been rarely reported [3]; hence references for this treatment are extremely limited. Here, we report the first case of triple-modality therapy to treat a patient with TAMOF in Korea, which involved the following therapeutic approaches: ECMO, CRRT, and TPE.
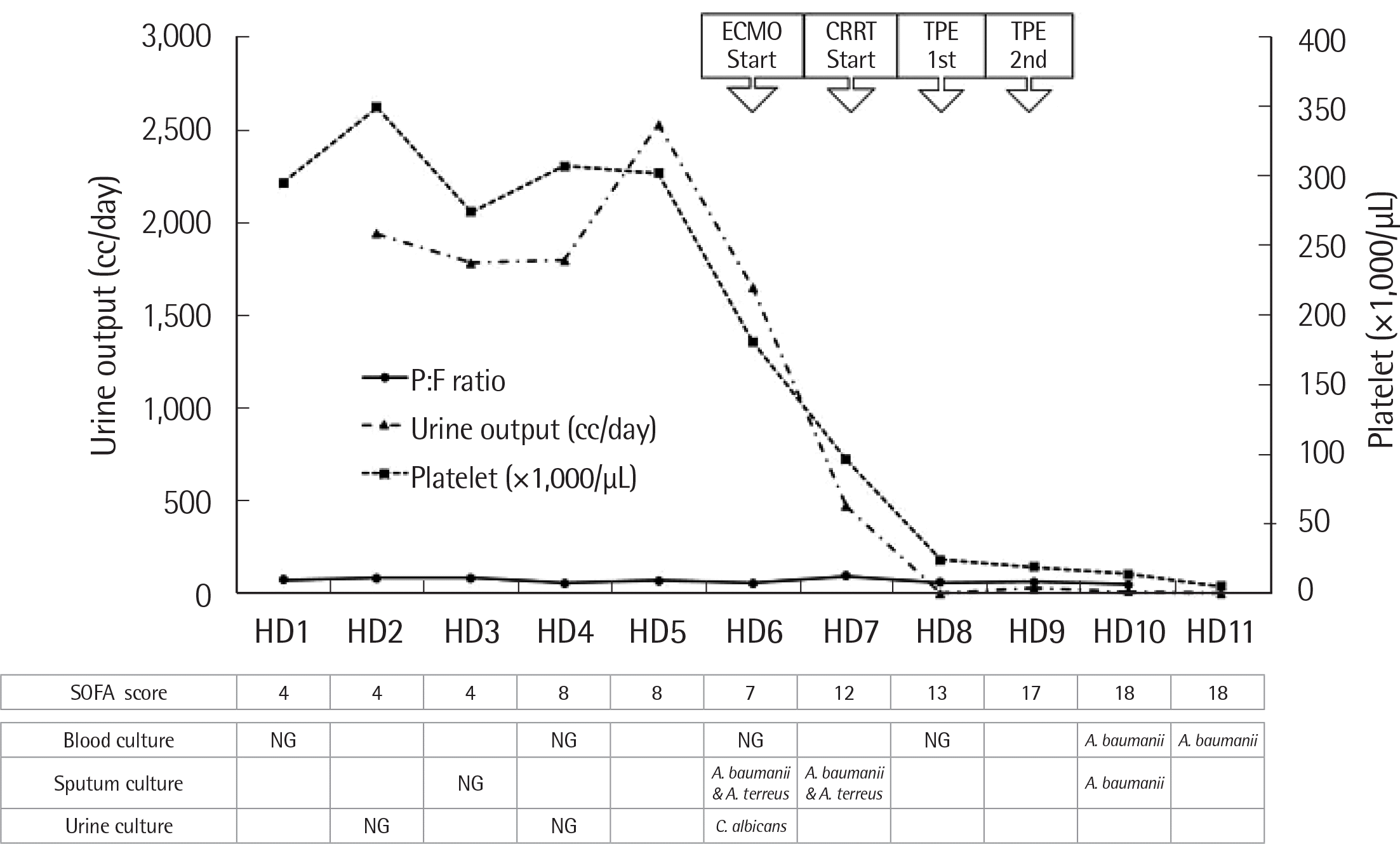
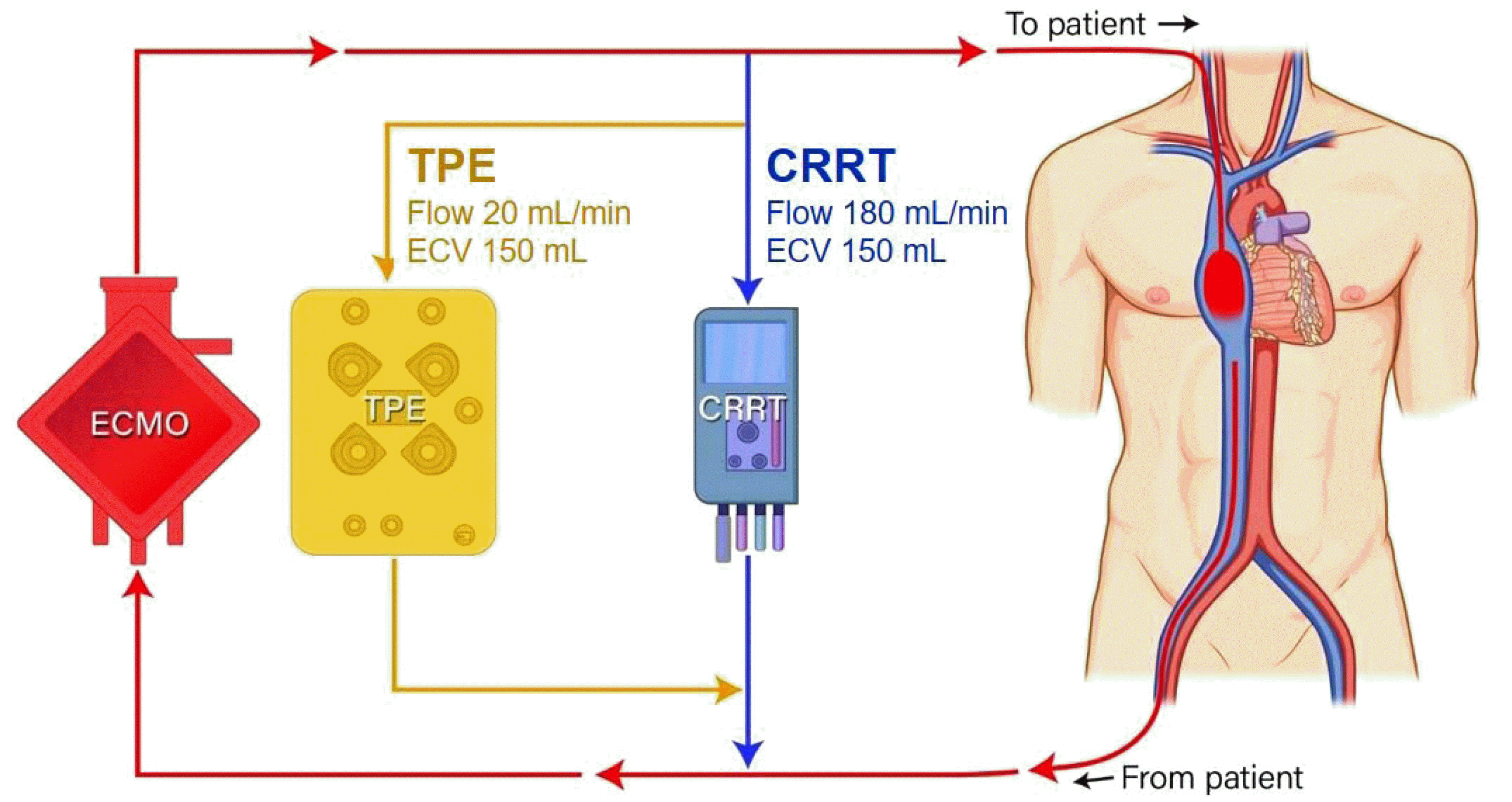
A 50-year-old woman diagnosed with organizing pneumonia was transferred to our institution. Initial arterial blood gas analysis revealed a PaO2/FiO2 ratio of 74. Chest computed tomography demonstrated aggravation of organizing pneumonia with lung injury. Under the impression of acute respiratory distress syndrome, intubation and mechanical ventilation were applied but failed to enhance SpO2 to the therapeutic level. ECMO was applied on hospital day (HD) 6 for oxygenation. Although the SpO2 had reached therapeutic status, the patient’s urine output decreased to less than 500 cc/day and serum creatinine level increased from 0.21 to 0.96 mg/dL on HD7. Under a presumed diagnosis of acute kidney injury combined with acute respiratory distress syndrome, CRRT was connected to the same circuit (Fig. 1, Fig. 2).
On HD7 the sequential organ failure assessment (SOFA) score for the diagnosis of sepsis increased from 7 to 12. On HD8, increased number of schistocytes (1-2 cells/high power field), decreased number of platelets (less than 50×109/L), and reduced ADAMTS13 activity (36%) led to the diagnosis of TAMOF.
As TPE was the treatment choice for TAMOF, a TPE circuit was connected to the previously set ECMO-CRRT circuit (Fig. 2) and simultaneous triple-modality therapy was performed on HD8 and HD9.
The calculated total blood volume was approximately 3,640 mL (56 kg×65 mL/kg) and the total extracorporeal volume was approximately 900 mL, which exceeded 15% of total blood volume. Initial inlet velocity was 30 mL/min, ACD-A to whole blood ratio was 1:15, and 16 units of fresh frozen plasma were used as replacement °uid. For three hours, one plasma volume of TPE was processed without adverse effect. Before TPE, the patient’s serum fibrinogen level had decreased to 80 mg/dL, while prothrombin time (PT) and activated partial thromboplastin time (APTT) were slightly prolonged to 15.2 sec and 55.7 sec, respectively. After two cycles of TPE, serum fibrinogen, PT, and APTT were normalized to 218 mg/dL, 12.7 sec, and 30.2 sec, respectively. On HD6, Acinetobacter baumanii and Aspergillus terreus were recovered from the patient’s sputum specimen. After the administration of anti-fungal therapeutics, the patient demonstrated no evidence of fungal infection, but A. baumanii was recovered from sputum and blood cultures on HD10. The general condition of the patient deteriorated and the SOFA score steadily increased; the patient expired on HD11.
Systemic endothelial microangiopathy after exposure to infection, autoimmune disease, transplantation, radiation, chemotherapy, and even cardiopulmonary bypass can lead to TAMOF. Based on this pathophysiology, TAMOF can be regarded as a thrombotic microangiopathic syndrome with a spectrum including thrombotic thrombocytopenic purpura (TTP), secondary thrombotic microangiopathy (TMA), and disseminated intravascular coagulation (DIC) [4], which can cause critical illness with unstable hemodynamics and organ dysfunction. The lung and kidney are the commonly insulted organs during TAMOF progression. So, timely application of extracorporeal organ supporting devices is required to maintain vital stability of the patient. ECMO and CRRT are crucial procedures that continuously replace failing cardiopulmonary and renal function, respectively. Usually, each of these procedure is performed individually at the bedside; however, depending on the condition of patients, both can be performed simultaneously with various connection methods for life support [5, 6].
Moreover, in TAMOF featuring TTP, secondary TMA, and DIC, TPE may be helpful in reducing mortality. In TTP, TPE has been proven to improve survival [7, 8] and accepted as first-line therapy with ‘Category I, Grade 1A’ in the American Society for Apheresis (ASFA) guidelines [9]. Further, in secondary TMA, TPE can be performed as first-line therapy, especially in complement- or drug-associated TMA, although more evidence regarding its efficacy is needed [9, 10]. In the case of DIC, although the usefulness of TPE has not been established, Nguyen and Carcillo suggested that TPE may be tried for removal of circulating tissue factor (TF) and plasminogen activator inhibitor type I (PAI-1), as well as theoretical restoration of antithrombin III and protein C in the plasma of patients [4].
TPE can be performed simultaneously with another extracorporeal organ supporting modality, including ECMO or CRRT. The simultaneous application of ECMO and TPE has been reported in both pediatric and adult patients with apparently effective and tolerable outcomes [11-13]. Hemodialysis, which is functionally similar to CRRT, could be combined with TPE simultaneously. Compared to sequential application, the advantages of the simultaneous application of different life support modalities include reduction of both total procedure time and anti-coagulant dose [14-17].
Depending on the severity of the illnesses, especially in case of TAMOF, patients may require simultaneous administration of EC-MO, CRRT, and TPE. In this case report, the patient was initially in a condition requiring ECMO-CRRT dual-application; however, several laboratory findings suggested the onset of TAMOF. An increase in the SOFA score by 2 points or more from the baseline, which is associated with an in-hospital mortality >10%, was observed around the time of new-onset thrombocytopenia [18]. Consequently, the addition of TPE to the ECMO-CRRT circuit was considered to relieve the unfavorable condition.
Triple-modality treatment is almost unprecedented, with only one previously reported study, wherein each of three pediatric TAMOF cases was treated using a TPE circuit connected to the ECMO-CRRT circuit; the treatment improved symptoms in the patients and eventually, all extracorporeal devices were disconnected [3]. However, this study did not describe the set-up of the whole circuit comprising combined modalities and the specific method for TPE. Therefore, we designed a new approach in this study.
Before performing TPE, the ECMO-CRRT dual-circuit was already set and the venous (outlet) line of the CRRT was connected ahead of the ECMO’s pump and oxygenator. This connection me-thod has two major advantages. First, purified blood in the venous (outlet) line of the CRRT can be easily returned into the drainage limb (inlet) line of the ECMO, which has negative pressure generated by the ECMO pump [5, 19]. Second, air bubbles or clots formed in the CRRT line may be trapped in the ECMO oxygenator before reentry into the patient [6].
We primarily focused on the connection of TPE to the ECMO-CRRT dual-circuit. As there was no previous reference, we focused on connecting the TPE to the CRRT line, rather than the ECMO line. We connected the access (inlet) line of the TPE to the arterial (inlet) line of the CRRT in parallel [14-17, 20]. As the pressure of fluid is inversely proportional to velocity, the TPE velocity was set slower than the CRRT velocity, resulting in increased pressure of the return (outlet) line of the TPE compared to that of the venous (outlet) line of the CRRT. This combined flow joined the drainage limb (inlet) line of the ECMO to achieve unilateral flow due to negative pressure in this line and removal of air bubbles or clots formed in the TPE and CRRT circuit, as shown in Fig. 2.
However, the recrudescence was faster than the therapeutic convalescence. From HD5 to HD7, the SOFA score increased from 8 to 12 and this deterioration could not be explained. Moreover, the progression of the disease could not be reversed by multiple extracorporeal life supports and sepsis was uncontrolled due to the spread of microbes from the sputum to the blood. Although TPE was applied at the point of thrombocytopenia, the SOFA score increased to 13. TAMOF seemed to occur irreversibly, which might explain the failure of the triple-modality treatment of extracorporeal life support.
Further studies on multiple application of extracorporeal life support are required for evaluating treatment effectiveness and intervention point during disease progression. Specifically, treatment of TAMOF with TPE and other extracorporeal life support requires further clinical studies for determining the intervention period, treatment number, and replacement fluids.
REFERENCES
1. Brun-Buisson C, Doyon F, Carlet J, Dellamonica P, Gouin F, Lepoutre A, et al. 1995; Incidence, risk factors, and outcome of severe sepsis and septic shock in adults: a multicenter prospective study in intensive care units. JAMA. 274:968–74. DOI: 10.1001/jama.1995.03530120060042. PMID: 7674528.

2. Venkata C, Kashyap R, Farmer JC, Afessa B. 2013; Thrombocytopenia in adult patients with sepsis: incidence, risk factors, and its association with clinical outcome. J Intensive Care. 1:9. DOI: 10.1186/2052-0492-1-9. PMID: 25810916. PMCID: PMC4373028.


3. Bridges BC, Hardison D, Pietsch J. 2013; A case series of the successful use of ECMO, continuous renal replacement therapy, and plasma exchange for thrombocytopenia-associated multiple organ failure. J Pediatr Surg. 48:1114–7. DOI: 10.1016/j.jpedsurg.2013.02.061. PMID: 23701790.

4. Nguyen TC, Carcillo JA. 2006; Bench-to-bedside review: thrombocytopenia-associated multiple organ failure - a newly appreciated syndrome in the critically ill. Crit Care. 10:235. DOI: 10.1186/cc5064. PMID: 17096864. PMCID: PMC1794442.


5. Seczyńska B, Królikowski W, Nowak I, Jankowski M, Szułdrzyński K, Szczeklik W. 2014; Continuous renal replacement therapy during extracorporeal membrane oxygenation in patients treated in medical intensive care unit: technical considerations. Ther Apher Dial. 18:523–34. DOI: 10.1111/1744-9987.12188. PMID: 25195931.

6. Chen H, Yu RG, Yin NN, Zhou JX. 2014; Combination of extracorporeal membrane oxygenation and continuous renal replacement therapy in critically ill patients: a systematic review. Crit Care. 18:675. DOI: 10.1186/s13054-014-0675-x. PMID: 25482187. PMCID: PMC4277651.


7. Rock GA, Shumak KH, Buskard NA, Blanchette VS, Kelton JG, Nair RC, et al. 1991; Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. New Engl J Med. 325:393–7. DOI: 10.1056/NEJM199108083250604. PMID: 2062330.

8. Brunskill SJ, Tusold A, Benjamin S, Stanworth SJ, Murphy MF. 2007; A systematic review of randomized controlled trials for plasma exchange in the treatment of thrombotic thrombocytopenic purpura. Transfus Med. 17:17–35. DOI: 10.1111/j.1365-3148.2006.00720.x. PMID: 17266701.

9. Padmanabhan A, Connelly-Smith L, Aqui N, Balogun RA, Klingel R, Meyer E, et al. 2019; Guidelines on the use of therapeutic apheresis in clinical practice - evidence-based approach from the Writing Committee of the American Society for Apheresis: The Eighth Special Issue. J Clin Apher. 34:171–354. DOI: 10.1002/jca.21705. PMID: 31180581.

10. Darmon M, Azoulay E, Thiery G, Ciroldi M, Galicier L, Parquet N, et al. 2006; Time course of organ dysfunction in thrombotic microangiopathy patients receiving either plasma perfusion or plasma exchange. Crit Cate Med. 34:2127–33. DOI: 10.1097/01.CCM.0000227659.14644.3E. PMID: 16763519.
11. Chong M, Lopez-Magallon AJ, Saenz L, Sharma MS, Althouse AD, Morell VO, et al. 2017; Use of therapeutic plasma exchange during extracorporeal life support in critically ill cardiac children with thrombocytopenia-associated multi-organ failure. Front Pediatr. 5:254. DOI: 10.3389/fped.2017.00254. PMID: 29250516. PMCID: PMC5716972.


12. Jhang J, Middlesworth W, Shaw R, Charette K, Papa J, Jefferson R, et al. 2007; Therapeutic plasma exchange performed in parallel with extra corporeal membrane oxygenation for antibody mediated rejection after heart transplantation. J Clin Apher. 22:333–8. DOI: 10.1002/jca.20151. PMID: 18080271.

13. Dyer M, Neal MD, Rollins-Raval MA, Raval JS. 2014; Simultaneous extracorporeal membrane oxygenation and therapeutic plasma exchange procedures are tolerable in both pediatric and adult patients. Transfusion. 54:1158–65. DOI: 10.1111/trf.12418. PMID: 24117674.

14. Dechmann-Sültemeyer T, Linkeschova R, Lenzen K, Kuril Z, Grabensee B, Voiculescu A. 2009; Tandem plasmapheresis and haemodialysis as a safe procedure in 82 patients with immune-mediated disease. Nephrol Dial Transplant. 24:252–7. DOI: 10.1093/ndt/gfn434. PMID: 18682492.

15. Pérez-Sáez MJ, Toledo K, Ojeda R, Crespo R, Soriano S, Álvarez de Lara MA, et al. 2011; Tandem plasmapheresis and hemodialysis: efficacy and safety. Ren Fail. 33:765–9. DOI: 10.3109/0886022X.2011.599912. PMID: 21770855.

16. Paglialonga F, Ardissino G, Biasuzzi A, Testa S, Edefonti A. 2012; Tandem plasma-exchange and haemodialysis in a paediatric dialysis unit. Pediat Nephrol. 27:493–5. DOI: 10.1007/s00467-011-2066-8. PMID: 22134881.
17. Farah M, Levin A, Kiaii M, Vickars L, Werb R. 2013; Combination hemodialysis and centrifugal therapeutic plasma exchange: 18 years of Canadian experience. Hemodials Int. 17:256–65. DOI: 10.1111/j.1542-4758.2012.00737.x. PMID: 22928813.
18. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. 2016; The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 315:801–10. DOI: 10.1001/jama.2016.0287. PMID: 26903338. PMCID: PMC4968574.


19. Sidebotham D, Allen SJ, McGeorge A, Ibbott N, Willcox T. 2012; Venovenous extracorporeal membrane oxygenation in adults: practical aspects of circuits, cannulae, and procedures. J Cardiothoac Vasc Anesth. 26:893–909. DOI: 10.1053/j.jvca.2012.02.001. PMID: 22503344.
20. Filler G, Clark WF, Huang SH. 2014; Tandem hemodialysis and plasma exchange. Pediatr Nephrol. 29:2077–82. DOI: 10.1007/s00467-013-2620-7. PMID: 24022368.

Fig. 1
Summary of disease progression in the TAMOF patient.
Abbreviations: ECMO, extracorporeal membrane oxygenation; CRRT, continuous renal replacement therapy; TPE, therapeutic plasma exchange; P:F ratio, PaO2:FiO2 ratio; HD, hospital day; SOFA, sequential organ failure assessment; NG, no growth; TAMOF, thrombocytopenia-associated multiple organ failure.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download