INTRODUCTION
The definition of sepsis has recently changed to include sequential organ failure assessment (SOFA) and quick SOFA (qSOFA) criteria, with an emphasis on organ failure [
1,
2]. The SOFA grade requires a complete assessment of numerous laboratory variables (a ratio of arterial oxygen partial pressure to fractional inspired oxygen platelets, bilirubin, mean arterial pressure, creatinine/urine output) and the Glasgow coma scale score. In contrast, qSOFA only requires three clinical criteria (respiratory rate, altered mentation, and systolic blood pressure) and can be applied as a rapid bedside screening tool. However, systemic inflammatory response syndrome (SIRS) remains an essential component in the diagnosis of sepsis [
3], and the measurement of biomarkers to detect exacerbations in inflammatory conditions could be helpful. Procalcitonin (PCT) is known to exhibit the highest accuracy among the many biomarkers of sepsis [
4]. In this study, we evaluated the diagnostic performance of PCT and qSOFA in combination, namely PCT–qSOFA, to propose a simpler and more accurate method of diagnosing sepsis.
Go to :

MATERIALS AND METHODS
1. Study population
Subjects who ordered more than two consecutive requests for blood culture (BC), owing to a suspicion of sepsis, and PCT at the same time between May 2016 and April 2017 were included in this study. Bloodstream infections due to Staphylococcus aureus, Enterococcus spp., Klebsiella pneumoniae, and Escherichia coli were included. The exclusion criteria were age <18 years, duplicate cases, and inadequate data for calculating SIRS or qSOFA scores.
The study population consisted of 102 patients with laboratory-confirmed primary bloodstream infection [
5] and 102 patients with repeated negative BC results. The criteria for laboratory-confirmed primary bloodstream infection were that the patient must have a recognized pathogen in one or more BCs and the organism cultured from the blood should not be related to an infection at another site. The recognized pathogens included
S. aureus,
Enterococcus spp.,
E. coli,
Pseudomonas spp.,
Klebsiella spp.,
Candida spp., and others.
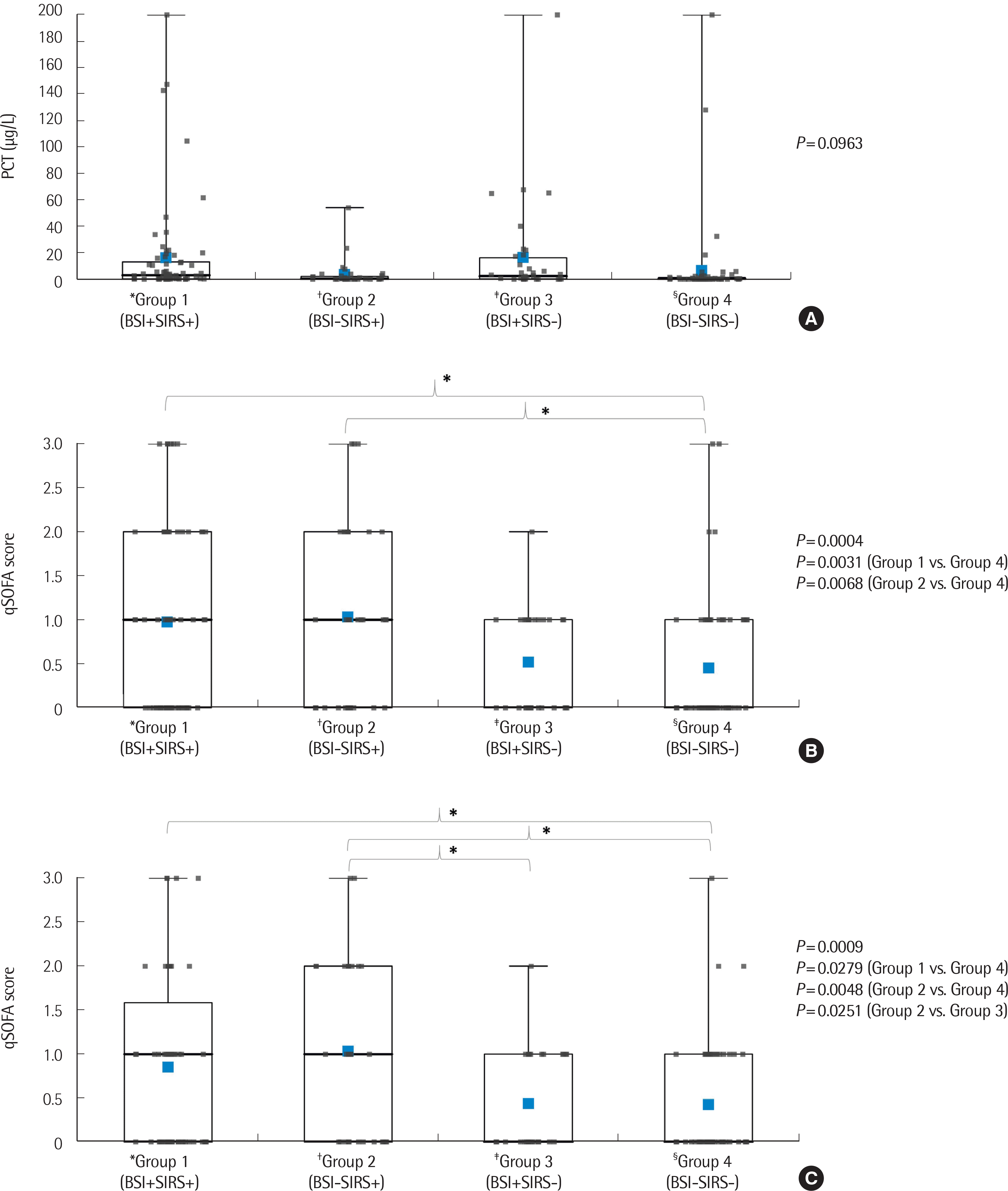
The 204 patients were subdivided into four groups on the basis of BC results and SIRS scores (≥2/4): the bacterial sepsis group (group 1, N=67), BC-negative sepsis group (group 2, N=37), BC-positive group without corresponding clinical symptoms or signs of sepsis (group 3, N=35), and the control group without evidence of sepsis (group 4, N=65). The main interest of this study was to determine whether patients outside the intensive care unit (ICU) as well as in the ICU can benefit from PCT and qSOFA assessment. Therefore, qSOFA scores were also divided into four groups, including and excluding patients in the ICU (N=23), in all the analyses.
This study was approved by the Institutional Review Board of National Health Insurance Service Ilsan Hospital (NHIMC 2018-05-014).
2. Laboratory diagnosis
BC was performed using BacT/ALERT FA/NA (bioMérieux, Marcy l’E’toile, France). Species identification and antimicrobial susceptibility testing were performed using the MALDI Biotyper system (Brucker Daltonics, Bremen, Germany) and Microscan WalkAway (Siemens Healthcare Diagnostics, Deerfield, IL, USA), respectively. PCT was measured using a chemiluminescence immunoassay (ADVIA Centaur, Siemens, Erlangen, Germany). Samples above the measurement range (0.02–75.00 μg/L) were diluted.
3. Review of clinical data
The total study population was subdivided into four groups based on BC results and SIRS scores. SIRS was scored for the day blood cultures were ordered by retrospectively reviewing electronic medical records. The criteria for SIRS were the presence of two or more of the following: temperature >38°C or <36°C, heart rate >90 beats/min, respiratory rate >20 breaths/min or a ratio of arterial oxygen partial pressure to fractional inspired oxygen <32 mmHg, white blood cell count >12,000/mm
3 or <4,000/mm
3, and immature bands >10% [
6]. The criteria for qSOFA were respiratory rate ≥22 breaths/min, altered mentation (Glasgow coma scale score <15), and systolic blood pressure ≤100 mmHg [
1]. We also obtained data such as age, sex, acquisition type, ICU admission, emergency room admission, diagnosis, previous antimicrobial use in the last seven days, prognosis, microbiology culture results, white blood cell count, mental status, Glasgow coma scale score, and vital signs. Mental status was categorized into three groups: alert, nearly alert–very drowsy, and stupor–coma. The mental status of “alert” was assigned when a patient could follow commands in a timely fashion. Stuporous patients required vigorous stimulation, and comatose patients could not respond appropriately to verbal nor painful stimuli. All the other patients were categorized into the nearly alert–very drowsy group.
4. Statistical analysis
Blood PCT levels and qSOFA scores were compared among the four groups using one-way ANOVA followed by the Bonferroni post hoc test.
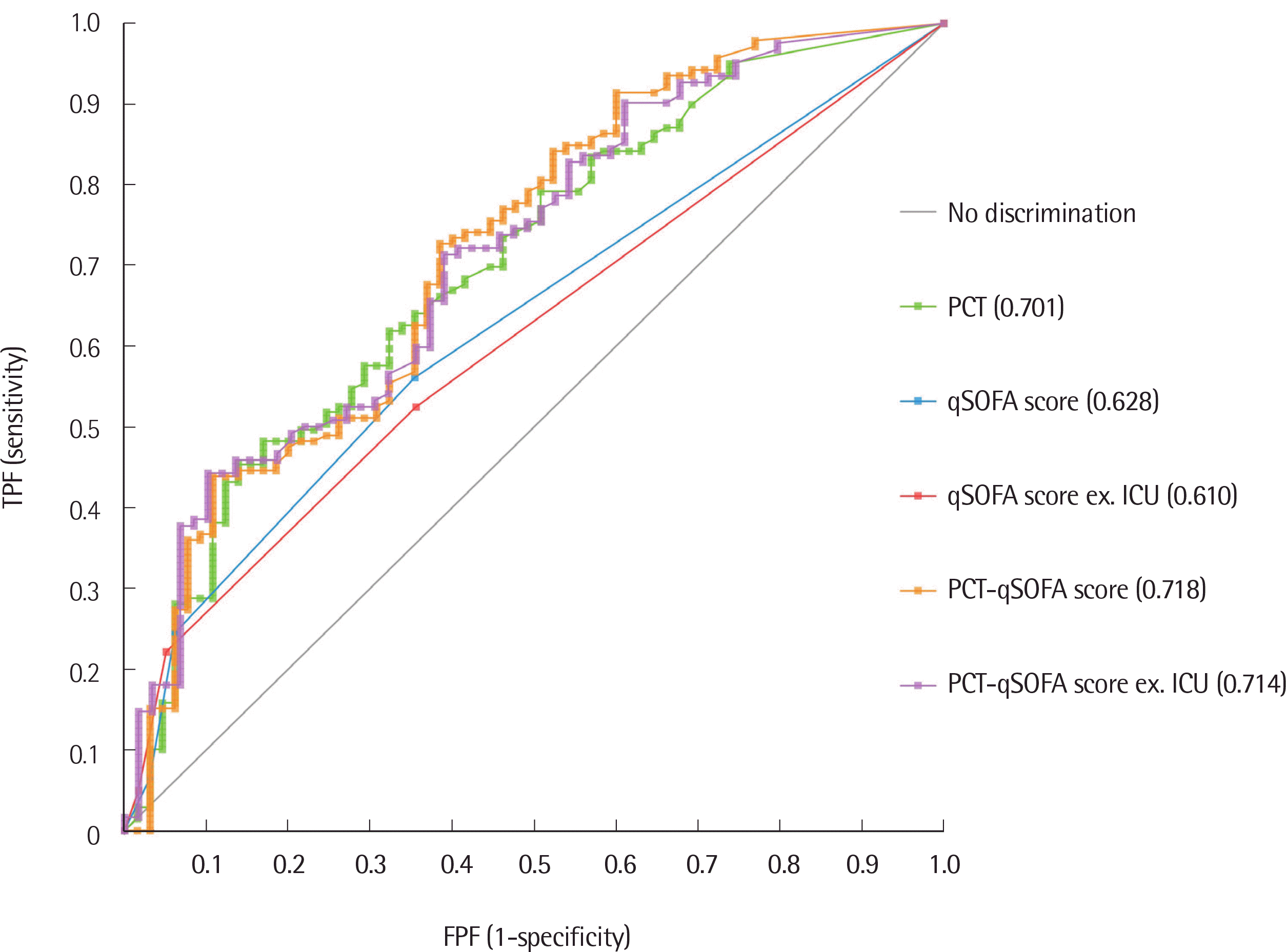
The Youden index, a summary of the receiver operating characteristic (ROC) curve analysis, was estimated to evaluate the diagnostic performance with logistic regression and area under the ROC (AUROC) curves. We used logistic regression analysis with the presence of sepsis as the binary dependent variable and PCT levels and qSOFA scores as predictor variables to calculate the predicted probability value of the combination of the two markers. This predicted probability value was also used to calculate the AUROC value for the combination of the two markers. Differences between the AUROCs were compared using the Delong method.
Post -test probability and post-test odds were analyzed to determine whether PCT–qSOFA improves the diagnostic value [
7]. We included group 3 in the sepsis group (groups 1, 2, and 3) in the post -test probability analysis. Group 3 corresponded to bloodstream infection according to the CDC standard [
5], and sepsis could not be completely excluded even though the criteria for SIRS were not met. Not all patients with SIRS are septic, and there are subgroups of hospitalized patients, particularly at extremes of age, who do not meet the criteria for SIRS on presentation but who progress to multiple organ dysfunction [
8].
Comparisons of SIRS and qSOFA scores in concordant and discordant groups were performed using the Mann–Whitney U test for continuous variables and the chi-square test for categorical variables. We used SAS software version 9.4 (SAS Institute, Cary, NC, USA), MedCalc version 19.4.1 (MedCalc, Mariakerke, Belgium) and Analyse-it Method Validation Edition software, version 4.60.4 (Analyse-it Software Ltd, City West Business Park, Leeds, UK) for the statistical analyses. P values less than 0.05 were considered significant.
Go to :

DISCUSSION
Sepsis is a severe disease and the primary cause of death from infection [
9]. The clinical and biological phenotypes of sepsis depend on the preexisting acute illness, long-standing comorbidities, medication usage, and interventions [
10]. Sepsis differs from infection in that it is an aberrant or dysregulated host response and is associated with organ dysfunction [
10].
The first definition of sepsis was based on SIRS and it included fever, tachypnea, hyperventilation, and leukocytosis [
3]. The newly introduced Sepsis-3 definition that was established in 2016 defined the word “sepsis” as life-threatening organ dysfunction caused by a dysregulated host response to infection [
1,
2]. However, the SOFA scoring system showed significant discrepancies when used to distinguish organ failure from infections [
11]. It was not easy to differentiate heterogeneous contexts, such as hypovolemia, severe heart failure, or severe pulmonary embolism, from sepsis using SOFA scores [
2], and the application of SOFA could delay the detection of sepsis before organ failure occurs.
The current study evaluated the combination of PCT and qSOFA rather than SOFA because evaluating the qSOFA score is easy for nurses and other medical workers who treat critical patients [
1]. PCT is a well-known indicator that is useful for the early confirmation of systemic inflammation and provides guidance for antibiotic therapy [
4]. There are many other biomarkers for sepsis, such as C-reactive protein (CRP), lactate, and cytokine levels. However, the CRP biomarker has a relatively low specificity [
4,
12]. Lactate concentration is inadequate for use as an early indicator, and cytokine levels do not show a significant advantage over PCT in this respect [
4,
12]. Moreover, the application of PCT–qSOFA may be more convenient if PCT is measured using a point-of-care device at the bedside [
13].
The diagnostic performance of qSOFA alone is not satisfactory. Therefore, some modifications are needed if qSOFA or PCT is to be applied for the diagnosis of sepsis. Post -test probability analysis was performed to define the diagnostic value of PCT–qSOFA. By definition, pretest probability is the probability of the disease before the test (prevalence, true positive plus false negative/total sample). In contrast, post -test probability is the probability of the disease after the test (positive predictive value, true positive/true positive plus false positive). A qSOFA score was proposed for the non-ICU setting; an initial retrospective analysis indicated that qSOFA scores could be a useful clinical tool, especially for physicians and other practitioners working outside the ICU [
1,
2]. The post-test probability of the combination of PCT and qSOFA scores remained the same after excluding patients in the ICU from the analysis. However, PCT–qSOFA scores of all patients showed improved post-test probability (from 0.772 to 0.884) and a positive likelihood ratio (from 1.59 to 3.55), implying that PCT–qSOFA detected 11.2% more sepsis events than qSOFA alone (88.4% vs. 77.2%).
Although this was the first attempt to combine PCT and qSOFA for the diagnosis of septicemia in Korea, other biomarkers are also needed to evaluate the diagnostic performance. Although the qSOFA score is known to be applicable to patients in the ICU, we attempted to apply it to all patients, including patients in the ICU, and found that PCT–qSOFA showed meaningful results with the modification of criteria from the presence of 2 or more qSOFA points to one.
There has been debate regarding suspicions of the diagnostic accuracy of SOFA and qSOFA on the basis of the Sepsis-3 definition [
14]. The new definition requires the presence of organ failure. Additionally, the SIRS criteria have been deleted altogether. Many heterogeneous contexts (for example hypovolemia, cardiac failure, and pulmonary embolism) were misdiagnosed as sepsis with qSOFA. On the contrary, hypoxemia, renal failure, coagulation defects, and hyperbilirubinemia could be missed with qSOFA. This may be the reason qSOFA was proposed for the non-ICU setting initially. We are of the opinion that PCT responds to systemic inflammatory response and compensates for qSOFA.
The limitation of this study is that it is a single-center analysis involving fewer subjects. A multicenter study is needed to secure more evidence. In conclusion, PCT–qSOFA is useful for evaluating sepsis in the ICU setting also. We recommend the application of PCT–qSOFA for the diagnosis of sepsis, regardless of ICU admission.
Go to :





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download