Abstract
Purpose
The purpose of this study was to investigate the effects of kartogenin (KGN) on the tendon-bone interface in a rat rotator cuff tear model.
Methods
Twenty male rats were divided into two equal groups; group 1 (repair only) and group 2 (KGN single injection). A rat rotator cuff rupture model was prepared, and KGN (500 μM) was injected into the repair site. The specimens were collected after 8 weeks, and biomechanical and histological analyses were performed. We assessed the healing quality of the tendon-to-bone repair site using six aspects of tendon tissue. The histological findings were classified semiquantitatively into four grades (grade 0, the poorest appearance; grade 1, poorer; grade 2, better; and grade 3, marked regeneration).
Results
Group 2 showed a higher mean ultimate load to failure than the control group (group 1: 25.78±31.38 N, group 2: 55.64±36.02 N; p=0.011). On histological analysis, group 2 exhibited a significantly greater total score (group 1: 7.20±2.14, group 2: 9.50±1.84; p=0.019), collagen fiber continuity (group 1: 1.20±0.42, group 2: 1.70±0.48; p=0.024), and collagen fiber density (group 1: 1.50±0.52, group 2: 2.20±0.63; p=0.080). Metachromasia were more intense in group 2 than in the control group.
Rotator cuff tears are an increasing problem as the incidence increases in the aging population and affect approximately 30% of people older than 60 years of age1. Although surgical treatment is one of the most common orthopedic operations, the failure rate of rotator cuff repair ranges from 11% to 90%2,3. The tendon-to-bone interface is the weakest link in rotator cuff repair, and many studies have demonstrated a high rate of incomplete healing at the tendon and bone interface4. Previous studies have focused on mechanical reinforcement of the tendon-to-bone insertion site and improvement of healing by administering various growth factors, including fibroblast growth factor and platelet-derived growth factor5,6. There have also been studies to apply stem cell therapy7,8. These techniques demonstrated some effect on the improvement of rotator cuff healing. Thus, in this study, we evaluated the different types of biochemical adjuvants that may affect the healing of the rotator cuff.
Kartogenin (KGN) (C20H15NO3; molecular weight, 317.3 g/mol) is an inducer that selectively differentiates human mesenchymal stem cells into cartilage cells9. KGN increases chondrocyte proliferation and induces cartilage differentiation in stem cells by increasing the levels of aggrecan, collagen II, and Sox-9, which are cartilage formation indicators10. In addition, KGN not only stimulates cartilage regeneration but also has a cartilage protective effect. KGN is a very stable small molecule, allowing it to be stored and transported at room temperature10. Even though a small amount of KGN was injected directly into the joints, there was no side effect in the experimental rats11.
Since its discovery, KGN has received attention as a new cartilage-forming drug for intra-articular treatment12. Although developed with a focus on osteoarthritis, it is currently being studied to promote the regeneration of other musculoskeletal disorders. Previous studies have shown that when KGN was injected in the Achilles tendon-bone junction of experimentally injured mice, the wound of the tendon-bone junction portion was regenerated by tissue formation9. Another study reported that the collagen composition of mouse dermis was stimulated to produce more collagen composition by KGN13. Such effects are expected to have a positive effect on the restoration process of the rotator cuff based on wound healing. However, there has not yet been much research regarding the effect of KGN in tendon-bone healing of rotator cuff tears. Therefore, the purpose of this study was to investigate the effects of KGN on the tendon-bone interface in a rat rotator cuff tear model. The hypothesis was that KGN would show increased fibrocartilage formation, superior collagen fiber organization, and increased biomechanical properties.
All animal procedures were approved by the Institutional Animal Commission at Kyungpook National University (No. KNU 2018-0083-1).
KGN (5 mg; Sigma-Aldrich, St. Louis, MO, USA) was prepared by dissolving 5 mg of KGN in 15 μL of dimethyl sulfoxide to make a 1,000 mM KGN stock solution, which was then diluted in phosphate-buffered saline (1×) to obtain a 1-mM KGN working solution. The final concentration was diluted to 500 μM, a concentration that has been used in the previous studies13.
Twenty male Sprague Dawley rats (weight, 300–350 g; 12 weeks old) were divided into two groups. Ten rats were randomly assigned to each group; group 1 (repair only, n=10) and group 2 (KGN single injection, n=10). The rats were anesthetized by intramuscular injection of zolazepam (15 mg/kg, Zoletil; Virbac S.A., Carroscedex, France) and xylazine hydrochloride (5 mg/kg, Rompun; Bayer HealthCare, Leverkusen, Germany). Both shoulders of the rat were shaved and sterilized with iodophor for aseptic conditions. A 3-cm longitudinal incision was made along the scapular spine. The deltoid muscle was split and the supraspinatus tendon exposed at the greater tuberosity. The supraspinatus tendon was isolated and transected the end of the tendon insertion site. Decortication of the foot print was performed with burr. Two bone tunnels were made using a drill at the greater tuberosity of the humeral head. The supraspinatus tendon was repaired with a single row through the bone tunnel with 3.0 Ethibond (Ethicon, Cincinnati, OH, USA). The control group (group 1) was subjected exclusively to repair. The experimental group (group 2; 500 μM of KGN) was injected in the bone- to-tendon interface (Fig. 1). Rats in both groups were sacrificed at 8 weeks after surgery. The right shoulder of each rat was used for biomechanical analysis, and the left was used for histological analysis.
At 8 weeks after surgery, each rat was euthanized with CO2 gas inhalation. The humeral head and proximal humeral-attached supraspinatus tendons were separated from both shoulders of each rat. We evaluated the biomechanical strength of the bone-to-tendon interface using a Universal Testing Machine (OTT-03; Oriental TM, Siheung, Korea). The specimen was measured to failure at a rate of 10 mm/sec with a 20-N load cell using a custom clamping system. The custom fixture clamping system for tensile test of the supraspinatus tendon consisted of two separate fixtures, namely a humeral head fixation unit, which rigidly fixed the humeral head and permitted the supraspinatus tendon and muscle attached to the humeral head to come out through a hole, and a cryogenic tendon fixation unit, which secured the myotendinous junction of the tendon using a sandpaper-attached clamp and liquid nitrogen to prevent slippage. The supraspinatus tendon was fixed to this system along its anatomic direction to allow tensile loading with the tendon-to-bone interface forming a right angle. The tensile load-to-failure data were automatically collected with a personal computer-based data acquisition system.
All specimens from both groups were fixed in 10 mL of sterile 10% formalin. After dehydration, they were embedded in paraffin and cut into 4-μm sections. The sections were stained with hematoxylin and eosin (H&E), Masson trichrome, picrosirius red, and toluidine blue staining.
We assessed the healing quality of the tendon-to-bone repair site using various aspects of tendon tissue. The score items included: (1) continuity of collagen fiber, (2) orientation of collagen fiber, (3) maturation of the tendon-to-bone interface, (4) collagen fiber density, (5) vascularity, and (6) cellularity. Each item was evaluated using a 4-point scoring system. For each item, the histological findings were classified semiquantitatively into four grades (grades 0–3). Grade 0 corresponded with the poorest appearance of the ruptured tendon, grade 1 indicated a poorer; grade 2 indicated a better grade, and grade 3 indicated a marked regeneration. Overall, the total score of a slide could range from 0 (ruptured tendon) to 18 (most marked regeneration)14. To eliminate observer error, all tissue slides were evaluated by a pathologist with at least 10 years of experience in a randomized, blinded fashion. All tissue slides were examined three times in the same position and area of rotator cuff tissue by microscope (Leica DMIL LED; Leica, Wetzlar, Germany) and image system (LAS V4.8; Leica) at ×50 magnification.
All statistical analyses were performed using SPSS ver. 12.0 software (SPSS Inc., Chicago, IL, USA). A p-value less than 0.05 indicated statistical significance. The Kruskal-Wallis test and post-hoc Mann-Whitney U-test were performed to evaluate the biomechanical and histological differences based on H&E staining between groups. Data are presented as the mean and standard deviation. Interclass correlation coefficients were used to assess intra- and interobserver reliability for histological analysis of the fat-to-muscle ratio.
Gross inspection showed no retear of the supraspinatus tendon-to-bone sites in both groups (p=0.531 at 8 weeks) (Table 1). However, in the biomechanical analysis, the prevalence of midsubstance tear was significantly higher in group 1 (p=0.01 at 8 weeks). Group 1 exhibited a greater tensile strain (group 1: 320.52±94.89%, group 2: 307.69±110.32%; p=0.784) and cross-section area (group 1: 4.23±1.17 mm2, group 2: 3.08±1.30 mm2; p=0.054) compared with group 1. However, there were no significant differences between the groups. Group 2 exhibited a greater ultimate failure load (group 1: 25.78±31.38 N, group 2: 55.64±36.02 N; p=0.011), ultimate stress (group 1: 5.96±5.95 N, group 2: 21.89±16.83 N; p=0.064), and stiffness (group 1: 3.44±5.59 N/mm, group 2: 6.62±4.21 N/mm; p=0.168). However, the ultimate failure load showed a significant difference (Table 2).
In the results of H&E staining (magnification, ×50), Masson’s trichrome staining, and picrosirius red staining (Fig. 2), group 2 exhibited a significantly greater total score at 8 weeks (group 1: 7.20±2.14, group 2: 9.50±1.84; p=0.019) compared with group 1. Collagen fiber continuity (group 1: 1.20±0.42, group 2: 1.70±0.48; p=0.024) and collagen fiber density (group 1: 1.50±0.52, group 2: 2.20±0.63; p=0.080) were significantly different in both groups. However, collagen fiber orientation (group 1: 1.30±0.48, group 2: 1.70±0.48; p=0.081), maturation of the tendon-to-bone interface structure (group 1: 1.30±0.48, group 2: 1.70±0.48; p=0.080), vascularity (group 1: 0.40±0.51, group 2: 0.10±0.31; p=0.135), and cellularity (group 1: 1.40±0.51, group 2: 1.70±0.82; p=0.342) demonstrated no significant differences between groups 1 and 2 (Table 3). The metachromasia area in toluidine blue staining was measured to confirm fibrocartilage at the tendon-to-bone junction. In group 2, the metachromasia appeared stronger and higher compared with group 1 (Fig. 3).
The purpose of this study was to investigate the effect of KGN on tendon-bone healing in a rat rotator cuff tear model. In this study, the KGN group showed more improved biomechanical properties and a histologically better healing quality in the bone-to-tendon interface than the control group. Johnson et al.15 reported that KGN, a small synthetic heterocyclic compound, was found to promote robust chondrogenic differentiation of primary human mesenchymal stem cells. After that, when KGN was injected intra-articularly into a mouse model of osteoarthritis, regeneration of the cartilage and prevention of joint degeneration was observed11,16. In 2014, Zhang et al.9 reported that when injected into intact rat patellar tendons and injured rat Achilles in vivo, KGN induced cartilage-like tissue formation in the tendon-to-bone junctions. Zhang et al.9,13 have studied the use of KGN in combination with platelet-rich plasma to facilitate fibrocartilage formation at the interface between tendon and bone of rat Achilles tendons. In their studies, KGN treatment was found to enhance production of collagens I and II and increase expression of Sox-9 and scleraxis, which is consistent with the formation of fibrocartilage-like tissue13. In addition, KGN treatment resulted in more organized collagen fibers and chondrocytes at the tissue interface, and KGN-treated tendon-to-bone constructs demonstrated greater mean ultimate strengths compared with the control group9,13.
Two previous studies reported on the effects of KGN in a rotator cuff tear model. Wang et al.17 evaluated the effect of KGN in augmenting healing of the repaired enthesis after rotator cuff repair in a murine model. They reported that superior collagen fiber organization and higher ultimate failure loads were seen in the KGN group at 4 weeks. Huang et al.18 compared the effects of repair only (control), microfracture+repair, and microfracture+ repair augmentation with a KGN-loaded gelatin methacryloyl (GelMA) hydrogel scaffold (combined) in tendon-to-bone healing in a rabbit rotator cuff tear model. The authors reported that the KGN-loaded GelMA hydrogel scaffolds group exhibited greater ultimate load to failure and stiffness than the other groups at 8 and 12 weeks after repair. In histological evaluation, the tendon maturation score of the KGN-loaded GelMA hydrogel scaffolds group was significantly higher than that of the other groups at 8 and 12 weeks after repair.
Our results were similar to previous studies. The tendons of the KGN group showed a greater mean ultimate load to failure than the control group, and KGN treatment appeared to enhance the formation and organization of collagen fibrils at the healing enthesis. This suggests that KGN has significant effects on collagen formation and organization at the interface between tendon and bone, even in a rotator cuff tear model. In addition, the two previous studies used scaffolds to minimize the diffusion of KGN into surrounding tissues and maximize the retainment of KGN. However, in our study, only a single injection of KGN was applied in KGN group. Compared with the control group, there was significant biomechanical and histological improvement in KGN group. Clinically single injection of the drug is the simplest method that can be further performed for healing after rotator cuff repair. It is considered important information on the usefulness of KGN to show a significant difference in effect through the simplest method. Therefore, we suggest that our research can provide basic information in other studies based on KGN.
In our study, the metachromasia area in toluidine blue staining was measured to confirm fibrocartilage at the tendon-to-bone junction. In the KGN group, the metachromasia appeared stronger and higher than in the control group. This suggests that KGN, which induces cartilage differentiation, promotes the production of fibrocartilage. Huang et al.18 also reported better fibrocartilage formation in the KGN group at 4, 8, and 12 weeks in toluidine blue staining compared with the control and microfracture+repair groups. However, Wang et al.17 reported that the mean percentage of area of fibrocartilage at the tendon-to-bone interface in Alcian blue staining was higher in the control group compared with the KGN group at 4 weeks. Regarding this difference, it seems that the histological evaluation system of fibrocartilage formation is semiquantitative and difficult to quantify. In the rotator cuff tear model, a significant increase in fibrocartilage formation can be observed at least 8 weeks after KGN injection.
It is believed that our study can be a foundation for future research on the use of KGN in rotator cuff tears. In previous cartilage defect models, various modalities such as chitosan nano- and microparticles16, photo-cross-linked scaffold with KGN- encapsulated nanoparticles19, and polyethylene glycol-modified poly (amidoamine) dendrimer20 have been used to enhance the effects of KGN. Future research will require evaluation of other formulations that can improve the effects of KGN in a rotator cuff tear model.
The current study had several limitations. First, in the experimental group, only a single dose of KGN was applied at the concentration suggested in a previous experiment. Therefore, it is not possible to know the dose-dependent effect of KGN, and information on the optimal concentration of it is not known. Second, because the grading system applied for histological evaluation was semiquantitative, there is a disadvantage that it is not objectively reliable. Third, the 8-week evaluation period may not be long enough for the final analysis, as there may be further change after 8 weeks. Further study with a longer evaluation period may be needed. Fourth, our tendon damage method is more like an acute rotator cuff tear than the chronic degenerative rotator cuff tears observed in most clinical patients.
In conclusion, a single-dose injection of KGN reinforces the biomechanical and histological properties at the tendon-to-bone interface in a rat rotator cuff tear model. Our study confirmed that KGN improves tissue regeneration and mechanical strength of rotator cuff tear. In previous studies, KGN has been widely evaluated as a remedy for cartilage regeneration, but the results of the current study confirm the tendon regeneration effect in a rotator cuff tear model.
REFERENCES
1. Yamaguchi K, Ditsios K, Middleton WD, Hildebolt CF, Galatz LM, Teefey SA. 2006; The demographic and morphological features of rotator cuff disease: a comparison of asymptomatic and symptomatic shoulders. J Bone Joint Surg Am. 88:1699–704. DOI: 10.2106/00004623-200608000-00002. PMID: 16882890.
2. Wang VM, Wang FC, McNickle AG, et al. 2010; Medial versus lateral supraspinatus tendon properties: implications for double- row rotator cuff repair. Am J Sports Med. 38:2456–63. DOI: 10.1177/0363546510376817. PMID: 20929937. PMCID: PMC3772634.
3. Le BT, Wu XL, Lam PH, Murrell GA. 2014; Factors predicting rotator cuff retears: an analysis of 1000 consecutive rotator cuff repairs. Am J Sports Med. 42:1134–42. DOI: 10.1177/0363546514525336. PMID: 24748610.
4. Castricini R, Longo UG, De Benedetto M, et al. 2011; Platelet-rich plasma augmentation for arthroscopic rotator cuff repair: a randomized controlled trial. Am J Sports Med. 39:258–65. DOI: 10.1177/0363546510390780. PMID: 21160018.
5. Wang LL, Yin XF, Chu XC, Zhang YB, Gong XN. 2018; Platelet-derived growth factor subunit B is required for tendon-bone healing using bone marrow-derived mesenchymal stem cells after rotator cuff repair in rats. J Cell Biochem. 119:8897–908. DOI: 10.1002/jcb.27143. PMID: 30105826.

6. Yonemitsu R, Tokunaga T, Shukunami C, et al. 2019; Fibroblast growth factor 2 enhances tendon-to-bone healing in a rat rotator cuff repair of chronic tears. Am J Sports Med. 47:1701–12. DOI: 10.1177/0363546519836959. PMID: 31038985.

7. Liu Q, Yu Y, Reisdorf RL, et al. 2019; Engineered tendon- fibrocartilage-bone composite and bone marrow-derived mesenchymal stem cell sheet augmentation promotes rotator cuff healing in a non-weight-bearing canine model. Biomaterials. 192:189–98. DOI: 10.1016/j.biomaterials.2018.10.037. PMID: 30453215.
8. Wang C, Hu Q, Song W, Yu W, He Y. 2020; Adipose stem cell- derived exosomes decrease fatty infiltration and enhance rotator cuff healing in a rabbit model of chronic tears. Am J Sports Med. 48:1456–64. DOI: 10.1177/0363546520908847. PMID: 32272021.
9. Zhang J, Wang JH. 2014; Kartogenin induces cartilage-like tissue formation in tendon-bone junction. Bone Res. 2:14008. DOI: 10.1038/boneres.2014.8. PMID: 25419468. PMCID: PMC4237211.

10. Im GI. 2018; Application of kartogenin for musculoskeletal regeneration. J Biomed Mater Res A. 106:1141–8. DOI: 10.1002/jbm.a.36300. PMID: 29164815.

11. Mohan G, Magnitsky S, Melkus G, et al. 2016; Kartogenin treatment prevented joint degeneration in a rodent model of osteoarthritis: a pilot study. J Orthop Res. 34:1780–9. DOI: 10.1002/jor.23197. PMID: 26895619. PMCID: PMC6348064.

12. Xu X, Shi D, Shen Y, et al. 2015; Full-thickness cartilage defects are repaired via a microfracture technique and intraarticular injection of the small-molecule compound kartogenin. Arthritis Res Ther. 17:20. DOI: 10.1186/s13075-015-0537-1. PMID: 25641548. PMCID: PMC4376363.

13. Zhang J, Yuan T, Zheng N, Zhou Y, Hogan MV, Wang JH. 2017; The combined use of kartogenin and platelet-rich plasma promotes fibrocartilage formation in the wounded rat Achilles tendon entheses. Bone Joint Res. 6:231–44. DOI: 10.1302/2046-3758.64.BJR-2017-0268.R1. PMID: 28450316. PMCID: PMC5415905.

14. Kim DH, Min SG, Yoon JP, et al. 2019; Mechanical augmentation with absorbable alginate sheet enhances healing of the rotator cuff. Orthopedics. 42:e104–10. DOI: 10.3928/01477447-20181206-04. PMID: 30540880.

15. Johnson K, Zhu S, Tremblay MS, et al. 2012; A stem cell-based approach to cartilage repair. Science. 336:717–21. DOI: 10.1126/science.1215157. PMID: 22491093.

16. Kang ML, Ko JY, Kim JE, Im GI. 2014; Intra-articular delivery of kartogenin-conjugated chitosan nano/microparticles for cartilage regeneration. Biomaterials. 35:9984–94. DOI: 10.1016/j.biomaterials.2014.08.042. PMID: 25241157.

17. Wang D, Tan H, Lebaschi AH, et al. 2018; Kartogenin enhances collagen organization and mechanical strength of the repaired enthesis in a murine model of rotator cuff repair. Arthroscopy. 34:2579–87. DOI: 10.1016/j.arthro.2018.04.022. PMID: 30037570. PMCID: PMC6371391.

18. Huang C, Zhang X, Luo H, et al. 2020; Jul. 7. Effect of kartogenin-loaded gelatin methacryloyl hydrogel scaffold with bone marrow stimulation for enthesis healing in rotator cuff repair. J Shoulder Elbow Surg. [Epub]. https://doi.org/10.1016/. DOI: 10.1016/j.jse.2020.06.013. PMID: 32650072.

19. Shi D, Xu X, Ye Y, et al. 2016; Photo-cross-linked scaffold with kartogenin-encapsulated nanoparticles for cartilage regeneration. ACS Nano. 10:1292–9. DOI: 10.1021/acsnano.5b06663. PMID: 26757419.

20. Hu Q, Ding B, Yan X, et al. 2017; Polyethylene glycol modified PAMAM dendrimer delivery of kartogenin to induce chondrogenic differentiation of mesenchymal stem cells. Nanomedicine. 13:2189–98. DOI: 10.1016/j.nano.2017.05.011. PMID: 28579434.

Fig. 1
The animal model and surgical procedures. (A, B) The deltoid muscle split and the supraspinatus tendon was exposed. (C) The supraspinatus tendon was isolated and transected at the end of the tendon insertion site. (D, E) Two bone tunnels were made at the greater tuberosity of the humeral head and single-row repair. The control group received repair only. (F) In the experimental group, kartogenin (500 μM) was injected into the bone-to-tendon interface.
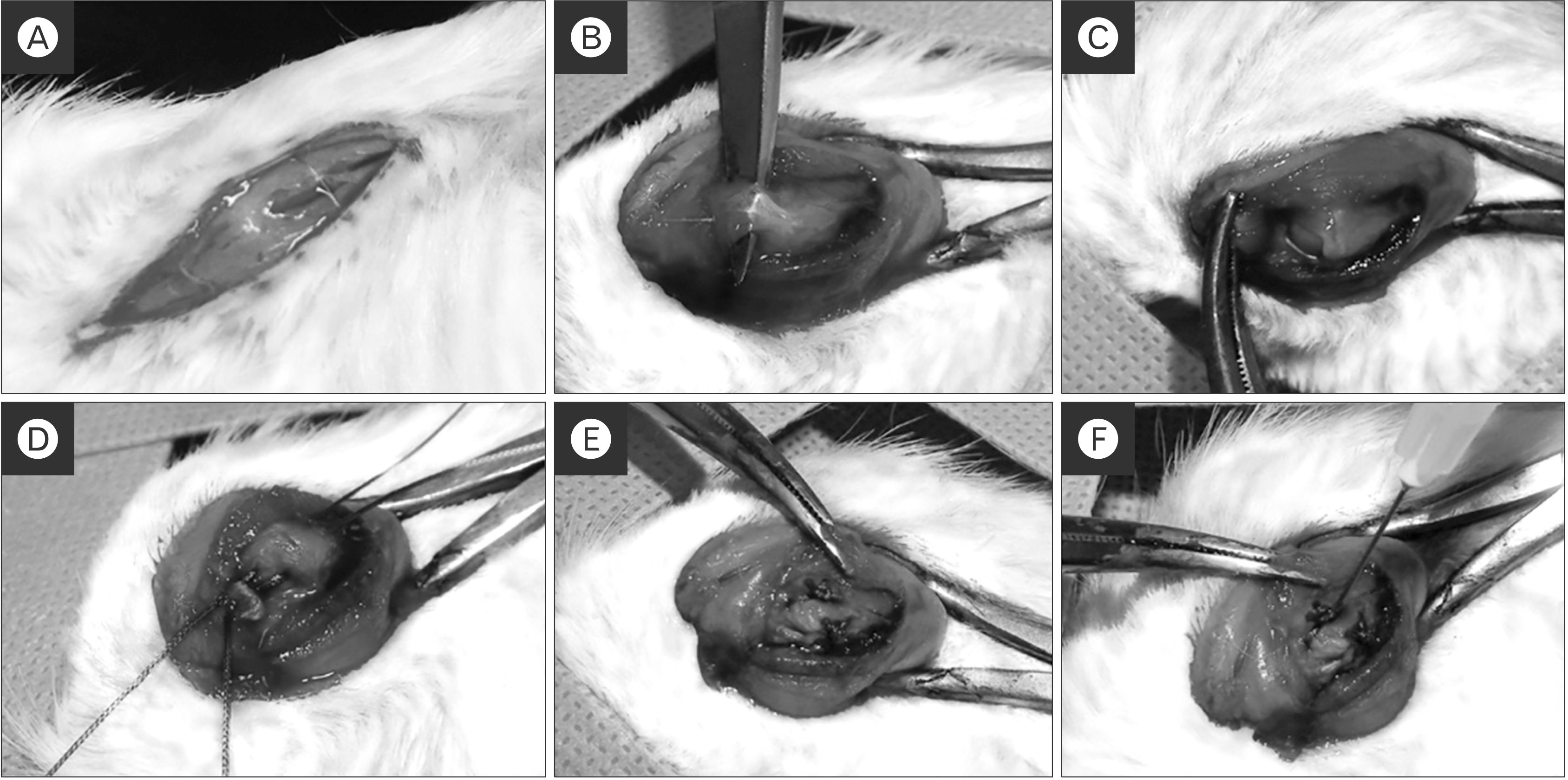
Fig. 2
Histological evaluation at 8 weeks after repair. Group 1 received only supraspinatus repair; group 2 received supraspinatus repair with a single injection of kartogenin. (A) H&E staining, ×50. (B) Masson trichrome staining, ×50. (C) Picrosirius red staining, ×50. Continuity, orientation, and density of collagen fiber showed a higher average grading in group 2 compared with group 1. Group 2 showed better maturation and density of the tendon-to-bone interface structure (arrows) than group 1.

Fig. 3
Histological evaluation at 8 weeks after repair. (A) Group 1 received only supraspinatus repair; (B) group 2 received supraspinatus repair with a single injection of kartogenin. The arrows indicate the tendon-to-bone interface. This area is the supraspinatus enthesis, and fibrocartilaginous transition site and was stained with Toluidine blue (×50). The metachromasia were more intense in the supraspinatus enthesis of group 2 compared to the control group.
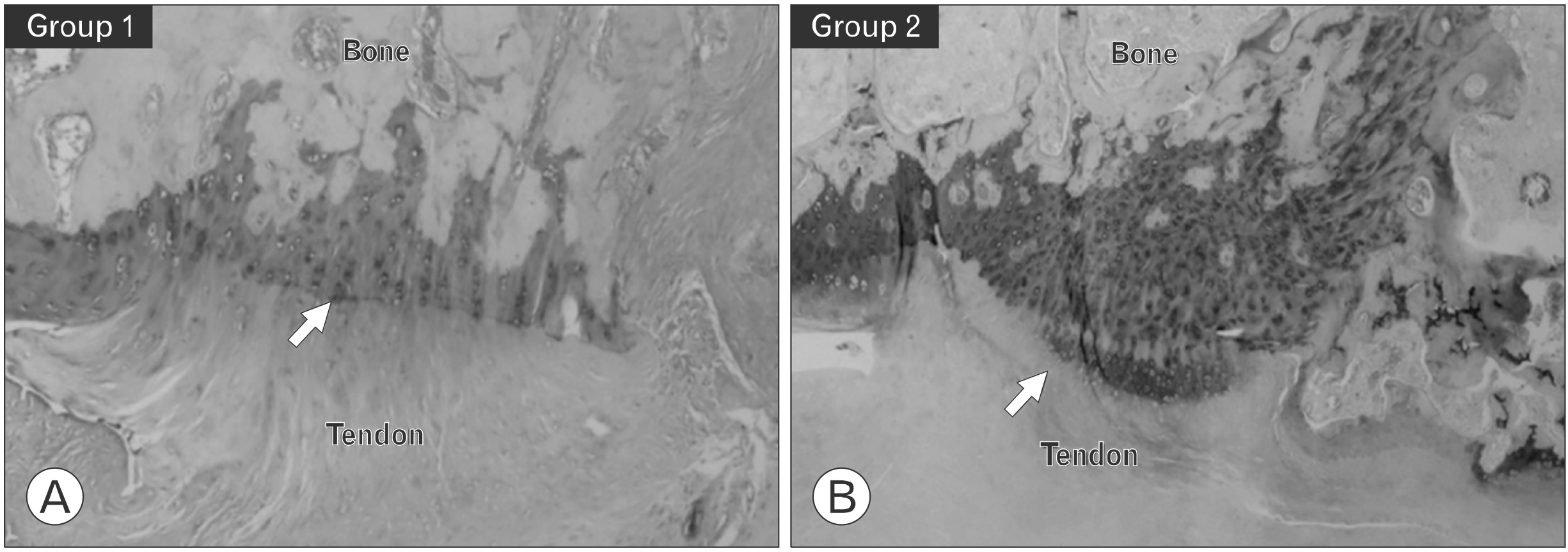
Table 1
Findings on gross inspection
| Variable | Group 1 (n=10) | Group 2 (n=10) | p-value |
|---|---|---|---|
| Retear | 0 | 0 | 0.531 |
| Bone-to-tendon failure | 3 | 9 | 0.01* |
| Midsubstance failure | 7 | 1 |
Table 2
Comparison of the biomechanical characteristics of both groups
| Variable | Group 1 | Group 2 | p-value |
|---|---|---|---|
| Cross-section area (mm2) | 4.23±1.17 | 3.08±1.30 | 0.054 |
| Tensile strain (%) | 320.52±94.89 | 307.69±110.32 | 0.784 |
| Ultimate failure load (N) | 25.78±31.38 | 55.64±36.02 | 0.011* |
| Ultimate stress (MPa) | 5.96±5.95 | 21.89±16.83 | 0.064 |
| Stiffness (N/mm) | 3.44±5.59 | 6.62±4.21 | 0.168 |
Table 3
Scoring of findings on histological analysis
| Variable | Group 1 | Group 2 | p-value |
|---|---|---|---|
| Collagen fiber continuity | 1.20±0.42 | 1.70±0.48 | 0.024* |
| Collagen fiber orientation | 1.30±0.48 | 1.70±0.48 | 0.081 |
| Collagen fiber density | 1.50±0.52 | 2.20±0.63 | 0.015* |
| Maturation of the tendon-to-bone interface structure | 1.50±0.70 | 2.10±0.73 | 0.080 |
| Vascularity | 0.40±0.51 | 0.10±0.31 | 0.135 |
| Cellularity | 1.40±0.51 | 1.70±0.82 | 0.342 |
| Total score | 7.20±2.14 | 9.50±1.84 | 0.019* |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download