Abstract
The coronavirus disease 2019 pandemic has caused a breakdown in the healthcare system worldwide. The need to rapidly update guidelines in order to control the transmission in the population and for evidenced-based healthcare care has led to the need for timely, voluminous and valid research. Amid the quest for a vaccine and better therapies, researchers clamouring for information has led to a wide variety of ethical issues due to the unique situation. This paper aims to examine the positive and negative aspects of recent changes in the process of obtaining informed consent. The article outlines the various aspects, from history, previously described exemptions to consenting as well as those implemented during the pandemic and the current impact of virtual methods. Further, the authors make recommendations based on the outcome of suggested adjustments described in the literature. This article looks into increasing the awareness of physicians and researchers about ethical issues that need to be addressed to provide optimal care for patients while assuring their integrity and confidentiality.
Go to : 
Graphical Abstract
Go to : 
As the world confronts coronavirus disease 2019 (COVID-19), optimizing patient care while reducing the risk of exposure and infection to patients and healthcare workers have led to the felt need for implementation of new technologies to tackle mounting pressures on the already stressed and crumbling healthcare system.12 The fast-paced creation of new protocols for healthcare delivery has led to different logistics and ethical issues to arise.34 In this article, the authors examine the positive and negative aspects of recent changes in the process of obtaining informed consent during the pandemic and further make recommendations based on the outcome of these suggested adjustments described in the literature, as well as propose ethical-based interventions for the future, with the ultimate goal of assuring optimal care of patients under appropriate moral standards.
Go to : 
Informed consent is not just a document, but an important component of patient-physician interaction in which the physician informs the patient about the intervention or treatment that will be performed, the step-by-step procedure, risks, benefits and alternative treatments. Taking consent is an ethical responsibility of the caregiver, is codified in the law, and is used in most healthcare institutions daily. However, taking a valid informed consent requires certain key requirements to be met, such as the cognitive ability of the patient to make a conscious decision, a transparent process with complete disclosure of information, and voluntary agreement without any coercion.5 Visualizing it, not only as a written and signed piece of paper but as a symbol of empathy, trust and confidence is crucial for a positive physician and patient interaction. This relationship is rooted in the ethical concept of beneficence. Over the last centuries, law and societal changes brought greater respect for autonomy and with it, greater emphasis on informed consent.5 This entails the right of the patient to receive information regarding research and/or procedures that he/she will be involved in.
Doctors should, indeed, consider individualizing the process of consenting and be able to do what is best for the patient by educating and discussing the most beneficial procedure or technique during extraordinary times. Nevertheless, the patient should not receive information regarding treatments not available; even if the evidence suggests higher efficacy.6 Transparency, as well as confidentiality and autonomy, are key components of the process of obtaining an informed consent and assuring patient well-being. A gap between theoretical goals and actual practice is noticeable and can be addressed in a wide variety of scenarios. For instance, “frailty” and dementia are present in an important percentage of the population of patients over 65 years old. Consenting in these patients is a true challenge due to the cognitive impairment present at the moment of the interview. Although there is not a specific technique for evaluating the capability of consenting, the National Institute of Health proposed several scores to determine the grade of dementia or cognitive affection in patients in long term care facilities.7 Exposure of the elderly to the health system under these circumstances mandates more rigorous attention from the physician, educating the patient at all time and respecting its autonomy. As recently described, COVID-19 could potentially cause intensive care unit delirium or other mental health affections. These issues often lead to negative outcomes in these patients such as permanent sequelae, prolonged hospital stay and increased rate of mortality.8
Another key area of concern is the conduct of procedures. Surgical procedures often present fragmentation in patient-surgeon interaction, mainly from poor disclosure during the process of obtaining informed consent. Most of these stem from miscommunication about the time between hospital arrival and time of intervention, not providing a copy of the document (which is, in fact, a legal right), potential technique change in the last minute, and, complications that can occur inside the operating room as well as in the postoperative period.9 Different investigations observed interesting results regarding the patient's thoughts and feelings before and after the given procedure. Gabay and Bokek-Cohen10 reported that in three large Israeli hospitals, more than half of the patients (n = 12) who received a life-saving surgery did not receive proper documentation or discussion before the intervention, even in some cases signing paperwork before receiving the appropriate information. Patients expressed distrust towards their surgeons due to lack of information regarding the postoperative level of pain, sense of depersonalization and loss of empathy. On the other side, research conducted at Mayo Clinic, in which 184 patients were asked whether they preferred to receive information about potential outcomes- postoperative visual loss after spine surgery, concluded that the majority (n = 158) of patients consider full disclosure a right and desire to receive the information completely, even in surgically-disabling consequences.11
During the ongoing COVID-19 pandemic, other vulnerable groups suffered exclusion as well as social and medical disparities, such as children and elderly. The inability to consent due to the public mandate for social isolation generates unique challenges to the health system. In sick patients, parents, caregivers or the patient's kin become the primary responsibility for decision-making and, under certain circumstances, stress and nervous states can derive on equivocations.12 Evidence suggests that COVID-19 affects elders with much more ease; efforts from governments worldwide towards equipping hospitals with COVID-19 care has led to a decrease in resources towards old age homes and support facilities. Hospital stay and isolation can create an undesirable environment for the patient's family and hospital staff, especially in those that require special care. Delicate decisions need to be made to secure the elderly's well-being. In this context, the fluidity of information between poles should be a high priority, and suggestions like implementing a team focused on the mental health in challenging settings could relieve the situation.13
Go to : 
Historic events in which the informed consent was not included as a key component of ethical medical practice lead to drastic consequences, such as the “Tuskegee Syphilis Study,” one that took place in Alabama in 1932 and was initially designed to last 6–8 months but lasted for 40 years. Approximately 600 African American men (400 men with early or late-stage syphilis and 200 men without the disease) volunteered to participate in such study after being told that they would receive the proper treatment. This never happened, and there was not an informed consent signed either. The experiment's hypothesis consisted on studying the course of syphilis in a particular community (poor rural African Americans with no access to health service) until death and observe whether the disease presents a different approach depending on the race.14 The global urgency to churn out a vaccine for COVID-19 puts the world at risks of repeating such mistakes once again. Developing a vaccine can be challenging, with its immunogenicity being fraught with dangers of autoimmune phenomena including demyelination and antibody-dependent enhancement as seen in dengue fever.15
Interestingly, there are always exceptions to the rule. In this case, certain scenarios in which informed consent waiver is allowed include; when the patient is incapacitated, in life-threatening emergencies with limited time to obtain informed consent and voluntary waiver consent. Besides, waiver of informed consent is permitted when research is directed towards the efficacy and safety of treatment of a particular disease in unusual conditions, such as the current pandemic. Recent publications address that due to the lack of knowledge regarding the pathophysiology of COVID-19 as well as it's histologic findings postmortem, the current pandemic should serve as a reason to waive the informed consent following deferred proxy approval in order to investigate and provide the health community with clear information.16
Go to : 
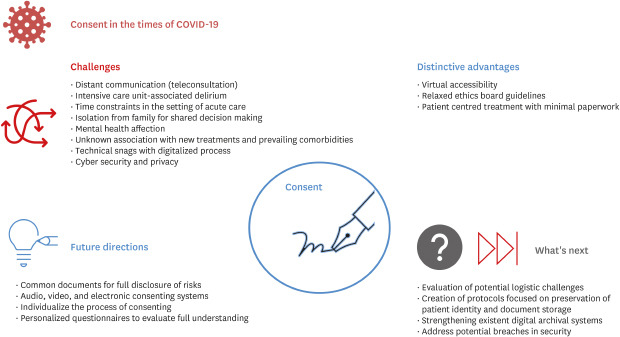
The informed consent and research itself have suffered changes during the pandemic; some of them impacting in the health system in a positive matter while others, not so much (Fig. 1).17 From a research point of view, the biggest impact is related to the shift of resource distribution towards clinical research, primarily focused on disease treatment, with intentions to bring relief to the pandemic.1819 Another important aspect to consider is the shortage of researchers and physicians, mainly due to the dual responsibility of providing treatment and care to patients while investigating.17 As far as the informed consent, changes in protocols and recommendations to decrease disease transmission generated tension between society and healthcare institutions. For instance, in clinical practice, patients and families might not understand or agree with decisions regarding triage; lack of cardiopulmonary resuscitation for all patients, if futile; postponed treatment of patients with disorders other than COVID-19. Treatment of COVID-19 often requires decisions to be made quickly, and some settings have been overwhelmed with patients needing urgent care, so there is less time for communication of information than usual.11 Another downside is that patients won't be able to take documents home to discuss with family members; patients who lack the capacity to consent for themselves face particular challenges.13
The process of obtaining informed consent can be, in certain circumstances, accompanied by a professional interest by a researcher/physician, leading towards an ethical misconduct in a medical practice setting due to a potential deviation of the principal purpose, which is to provide care and treatment to the patient. On the other side, the barriers imposed by the pandemic, such as the decrease in available hospital beds for non COVID-19 patients, as well as the potential issues derived from teleconsultations, like the lack of person-person communication and inability to correctly evaluate full understanding of the information provided by the physician, led to a direct relation between the arising of ethical issues and the process of consenting. Another key point is the challenge brought with the ethical responsibility from healthcare providers of maintaining patient's confidentiality; while remarking the importance of communication in order to protect other members of the society.20
Therefore, a careful evaluation of prevailing ethical standards and their addressal would be useful in the endeavors to devise new guidelines for secure and correct medical practice.21 In order to prevent such medical errors, these should focus mainly on key points such as: 1) Integrity: Evaluate what is known and what is not about the impact of COVID-19 on mental health to assure complete understanding of the information and avoid professional research benefit only. 2) Justice and equity: Resource and attention distribution emphasized towards those in disadvantage or need assuring well-being to the community as a whole. 3) Preservation of patient's privacy: Respect the patient's dignity and role in their community, by securing their privacy.
Isolation from the family while admitted with COVID-19 can have an adverse impact on the mental health of patients, which may reflect in reluctance to participate and consent to research. Not less important is the fact that there is a variable risk of infection transmission from the unsuspected infected members of the surgical team as well as administrative staff to the patient during surgical or invasive procedures as well as direct contact. The level and frequency of exposure that should be allowed is another glaring question that faces administration all over the world. At the same time, healthcare institutions struggle to keep up with the surge in cases amid a shortage of healthcare workers. Also, little is known about the association and interaction of this novel disease with other comorbidities, drug treatments, as well as the potential outcomes, a situation with a reasonable risk of fallacious advice due to natural urge of a physician to engage in the act of commissions rather than omission under dire and pressing circumstances.
Go to : 
On the positive side, it is important to acknowledge that the high infectivity of COVID-19 has forced the health community to adapt to new ways of interaction quickly.22 The use of existing technologies has led to a decrease in the number of patients attending hospital facilities, where they could easily be exposed. Teleconsultations are being used for delivering healthcare. However, the length of time for which routine healthcare and a formal physical examination can be ethically deferred remains to be established. While COVID-19 and acute care take precedence, with the long projected pandemic duration, it is vital to determine ways and means to deliver routine care, including ancillary services which are an essential component of wholesome medical care.
Access to teleconsultations has been eased by widespread access to the Internet and newer apps, which has made certain procedures cheaper.23 Electronic informed consent (EIC) has been tested as a possible method to consent while preserving the patient's integrity. Hussein et al., 2017 concluded that the use of smartphones enhanced enrollment of patients in time-sensitive clinical research such as DAWN (Acute Stroke Trial) as well as other interventions. The methodology primarily consisted of a constant interaction between health care providers and legally authorized representatives, in which, via text message, the appropriate link was provided to be filled alongside a freehand electronic signature.24 One of the newest proposed and used way of obtaining informed consent is through audio-visual devices. With it, comes a world of uncertainty and doubt. Different perspectives need to be discussed. Recently, the Food and Drug Administration of the United States has laid out 13 ground rules for an electronic signature to be valid.25 Primary data checks by institutional review boards may be useful to ensure compliance with prevailing standards when resorting to EIC procedures.26
Go to : 
From an ethical aspect, transparency is an important factor for the patient's well-being. In this case, the benefit is related to the fact that every part of the process is being recorded which would indeed increase society's confidence in the health system and clinical trials. Physician-patient interaction will not be compromised if ethical standards are being met; the use of personalized questionnaires could then evaluate the complete understanding of the intervention once the interview concludes to assure the unconditional approval by the patient. Novel evidence demonstrates that there is no significant difference in patient comprehension via EIC in contrast to conventional informed consent in clinical trials.27 These adjustments could potentially change the way we conduct research studies or provide treatment because of a possible shift from heavy documentation with protocols that patients may not comprehend to focusing on true understanding and voluntary involvement.
A few potential issues should be mentioned, as well. We could title them as operational challenges that attempt against ethics; for example, infrastructure, language barrier and poor image/audio quality, to name a few. Two important conditions should be highlighted; first, the storage and archival of the documents.
Everybody who participates in obtaining consent should be correctly prepared and be aware of the location of the files. Investigators/physicians should avoid misuse or theft, and with it, patient's confidentiality. The second point includes the preservation of patient identity being compromised. The widespread use of teleconsultations has led to a visible shift to smartphone app-based research, brining specific aspects under consideration.28 Recently, the “Aarogya Setu” from India was launched to assist self-assessment, contact tracing and health messages by the Indian government. In this specific case, over 50 million people who downloaded the app as part of a government mandate were informed that data was only shared with the government, but without mentioning a specific department. The public mandate to download the app as well as it's requirements such as continuous turned-on Bluetooth and location tracking has raised concerns in the Indian population.29 Under the Disaster Management Act, which enables Indian states to act “as necessary,” concerns were raised on the disruption of privacy obligations, leading to stigmatization from neighbours and employers, without any solution to these patients. On the other side, there is no specific time limit for personal information being exposed while using social media. As a consequence, the Indian government has been challenged in court because of these infractions. Fortunately, actions have taken place, and few states acted on the Indian Council of Medical Research and claimed to support patient's rights, and those who attempt against it will face legal consequences.30
Go to : 
To conclude, a lot needs to be further investigated to manage the ongoing pandemic better. The short time for communication during patient evaluation and the lack of evidence regarding disease course and treatment created tension between patients and physicians. The limited evidence available might not be sufficient to make an informed decision. The creation of protocols, designed to prevent the dissemination of the infection, particularly impacted certain groups in a negative way. For instance, a large amount of hospitals have seriously restricted visitation to facilities; efforts should be directed towards continuing to support pediatrics, elders and patients with intellectual and developmental disabilities, due to the inability to consent and express their needs.
On the other side, the use of virtual methods to consent and provide optimal coverage to patients has lowered the exposure and interaction between the healthcare system and the general population, decreasing the risk of viral transmission by preventing any sort of physical contact, while providing care to the community. In the modern era, where the majority of the society has access to Internet, telecommunication has helped to overcome the barriers caused by isolation; patients with non-critical diseases can have access to therapies, without the need to attend a medical facility. For example, those in need of psychological or psychiatric attention.31
Despite benefits outweighing risks, human rights should be respected at all times. An expanded armamentarium of digital tools to support informed consent paves the way for better communication between health care professionals and patients. Hopefully these will herald the onset of a new era of digitalized ethical consent-driven evidence-based medicine. In order to do so, we recommend institutions to create questionnaires to evaluate full understanding after receiving pertinent information; to individualize the process of consenting, considering patient's age, level of education, mental health status, etc.), to implement audio, visual and EIC after educating health-care providers about ethical issues, how should they be prevented and managed. Journal editors may play a larger role by conducting active checks to add ethics statements in published articles. While current regulations allow waivers of consent in certain emergencies, greater elaboration of the gray areas may define a better future for ethical research.
Go to : 
ACKNOWLEDGMENTS
The authors thank Prof. Armen Gasparyan, Dr. Malke Asaad and Connecting Researchers for their help with this work.
Go to : 
References
1. Whitelaw S, Mamas MA, Topol E, Van Spall HG. Applications of digital technology in COVID-19 pandemic planning and response. Lancet Digit Health. 2020; 2(8):e435–40. PMID: 32835201.

2. Monaghesh E, Hajizadeh A. The role of telehealth during COVID-19 outbreak: a systematic review based on current evidence. BMC Public Health. 2020; 20(1):1193. PMID: 32738884.



3. Gupta L, Gasparyan AY, Zimba O, Misra DP. Scholarly publishing and journal targeting in the time of the coronavirus disease 2019 (COVID-19) pandemic: a cross-sectional survey of rheumatologists and other specialists. Rheumatol Int. 2020; 40(12):2023–2030. PMID: 33048199.


4. Bryan AF, Milner R, Roggin KK, Angelos P, Matthews JB. Unknown unknowns: surgical consent during the COVID-19 pandemic. Ann Surg. 2020; 272(2):e161–2. PMID: 32675526.

5. Cocanour CS. Informed consent-it's more than a signature on a piece of paper. Am J Surg. 2017; 214(6):993–997. PMID: 28974311.


6. Turnham HL, Dunn M, Hill E, Thornburn GT, Wilkinson D. Consent in the time of COVID-19. J Med Ethics. 2020; 46(9):565–568. PMID: 32522812.



7. Tori K, Kalligeros M, Shehadeh F, Khader R, Nanda A, van Aalst R, et al. The process of obtaining informed consent to research in long term care facilities (LTCFs): an Observational Clinical Study. Medicine (Baltimore). 2020; 99(21):e20225. PMID: 32481294.
8. Silbert BS, Scott DA. Informed Consent in Patients With Frailty Syndrome. Anesth Analg. 2020; 130(6):1474–1481. PMID: 32384337.


9. Zahrai A, Bhanot K, Mei XY, Crawford E, Tan Z, Yee A, et al. Surgeon clinical practice variation and patient preferences during the informed consent discussion: a mixed-methods analysis in lumbar spine surgery. Can J Surg. 2020; 63(3):E284–E291. PMID: 32437095.

10. Gabay G, Bokek-Cohen Y. Infringement of the right to surgical informed consent: negligent disclosure and its impact on patient trust in surgeons at public general hospitals - the voice of the patient. BMC Med Ethics. 2019; 20(1):77. PMID: 31660956.



11. Corda DM, Dexter F, Pasternak JJ, Trentman TL, Nottmeier EW, Brull SJ. Patients' perspective on full disclosure and informed consent regarding postoperative visual loss associated with spinal surgery in the prone position. Mayo Clin Proc. 2011; 86(9):865–868. PMID: 21878598.



12. Misra DP, Agarwal V. Real-world evidence in rheumatic diseases: relevance and lessons learnt. Rheumatol Int. 2019; 39(3):403–416. PMID: 30725156.


13. McGuire AL, Aulisio MP, Davis FD, Erwin C, Harter TD, Jagsi R, et al. Ethical challenges arising in the COVID-19 pandemic: an overview from the association of bioethics program directors (ABPD) task force. Am J Bioeth. 2020; 20(7):15–27. PMID: 32511078.


14. Paul C, Brookes B. The rationalization of unethical research: revisionist accounts of the tuskegee syphilis study and the New Zealand “Unfortunate Experiment”. Am J Public Health. 2015; 105(10):e12–9.

15. Misra DP, Agarwal V. To Act…….or to wait for the evidence: Ethics in the time of covid-19! Indian J Rheumatol. 2020; 15(1):3–4.

16. Moodley K, Allwood BW, Rossouw TM. Consent for critical care research after death from COVID-19: arguments for a waiver. S Afr Med J. 2020; 110(7):629–634. PMID: 32880337.

17. Panda PK, Stockler MR, Gulia A. Clinical research during coronavirus disease pandemic: challenges and way forward. Indian J Med Sci. 2020; 72(2):101–106.


18. AlNaamani K, AlSinani S, Barkun AN. Medical research during the COVID-19 pandemic. World J Clin Cases. 2020; 8(15):3156–3163. PMID: 32874970.



19. Tuttle KR. Impact of the COVID-19 pandemic on clinical research. Nat Rev Nephrol. 2020; 16(10):562–564. PMID: 32760016.


20. Kramer JB, Brown DE, Kopar PK. Ethics in the time of coronavirus: recommendations in the COVID-19 pandemic. J Am Coll Surg. 2020; 230(6):1114–1118. PMID: 32278728.



21. Chenneville T, Schwartz-Mette R. Ethical considerations for psychologists in the time of COVID-19. Am Psychol. 2020; 75(5):644–654. PMID: 32437180.


22. Kichloo A, Albosta M, Dettloff K, Wani F, El-Amir Z, Singh J, et al. Telemedicine, the current COVID-19 pandemic and the future: a narrative review and perspectives moving forward in the USA. Fam Med Community Health. 2020; 8(3):e000530. PMID: 32816942.

23. Gupta L, Misra DP, Agarwal V, Balan S, Agarwal V. Response to: ‘Telerheumatology in COVID-19 era: a study from a psoriatic arthritis cohort’ by Costa et al. Ann Rhem Dis. Forthcoming 2020. DOI: 10.1136/annrheumdis-2020-217953.

24. Haussen DC, Doppelheuer S, Schindler K, Grossberg JA, Bouslama M, Schultz M, et al. Utilization of a smartphone platform for electronic informed consent in acute stroke trials. Stroke. 2017; 48(11):3156–3160. PMID: 28986425.


25. U.S. Food and Drug Administration. Part 11, Electronic records; electronic signatures - scope and application. Updated September 2003. Accessed November 21, 2020. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/part-11-electronic-records-electronic-signatures-scope-and-application.
26. Misra DP, Agarwal V. Blaming the peer reviewer: don't shoot the messenger!! Indian J Rheumatol. 2020; 15(3):162–164.
27. Bobb MR, Van Heukelom PG, Faine BA, Ahmed A, Messerly JT, Bell G, et al. Telemedicine provides noninferior research informed consent for remote study enrollment: a randomized controlled trial. Acad Emerg Med. 2016; 23(7):759–765. PMID: 26990899.



28. Gupta L, Chinoy H. Monitoring disease activity and damage in adult and juvenile idiopathic inflammatory myopathy. Curr Opin Rheumatol. 2020; 32(6):553–561. PMID: 32890032.


29. Davalbhakta S, Advani S, Kumar S, Agarwal V, Bhoyar S, Fedirko E, et al. A systematic review of smartphone applications available for corona virus disease 2019 (COVID19) and the assessment of their quality using the mobile application rating scale (MARS). J Med Syst. 2020; 44(9):164. PMID: 32779002.



30. Sneha P. Do India's COVID-19 patients have a right to privacy? Updated July 2020. Accessed November 8, 2020. https://science.thewire.in/health/do-indias-covid-19-patients-have-a-right-to-privacy/.
31. Fortney JC, Pyne JM, Edlund MJ, Williams DK, Robinson DE, Mittal D, et al. A randomized trial of telemedicine-based collaborative care for depression. J Gen Intern Med. 2007; 22(8):1086–1093. PMID: 17492326.



Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download