In April 2020, a UK pediatric report of an unusual inflammatory illness led to similar cases being rapidly reported in other countries.
12 The illness was referred to as multisystem inflammatory syndrome in children (MIS-C) by the US Centers for Disease Control and Prevention and World Health Organization. After the initial report, similar cases were described in clusters in places where coronavirus disease 2019 (COVID-19) has been widely prevalent.
34 Notably, despite clinical manifestations that mimicked Kawasaki disease (KD) and the fact that KD is more common among Asian ethnicities, there had been no report of MIS-C from Asian countries, including China.
5
On October 5, 2020, however, the Ministry of Health and Welfare of Korea announced the first two cases of MIS-C in Korea.
6 One of those cases, as described in this article, had evidence of molecularly diagnosed COVID-19 infection. The patient did not present with the characteristics of KD and did not require intensive care unit care. Nevertheless, we encountered and overcame several challenges in the diagnosis and management of MIS-C in this case. We would like to share some lessons with others that could help save lives.
On September 14, 2020, a 12-year-old boy visited the emergency department (ED) of Chungbuk National University Hospital (CBNUH) with chief complaints of fever and abdominal pain that started two days and a day before, respectively. His past medical history included a hospital stay at CBNUH from August 19 to September 1 for a diagnosis of COVID-19 by contact tracing and polymerase chain reaction test. He remained asymptomatic throughout his previous admission. In the ED, the patient also complained of headache and nausea. His vital signs were as follows: blood pressure, 127/62 mmHg; body temperature, 39.5°C; pulse rate, 103 beats/min; and respiratory rate, 22 breaths/min. He was alert and had no palpable lymph nodes (LNs) in the cervical, axillary, abdominal, and inguinal regions. Abdominal tenderness in the epigastric and right lower quadrant (RLQ) of the abdomen was noticed; however, rebound tenderness was not definite.
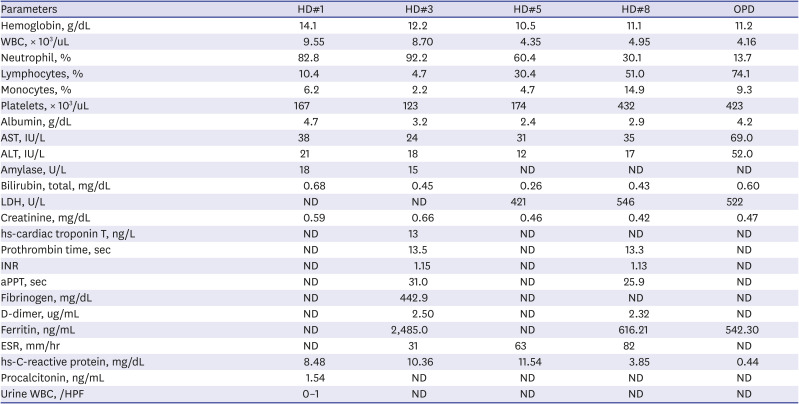
Complete blood cell count results were as follows: white blood cells, 9.55 × 10
3/uL (segmented neutrophil, 82.8%); hemoglobin, 14.1 g/dL; and platelets, 167 × 10
3/uL. His C-reactive protein level was 8.48 mg/dL (normal range, 0–0.3 mg/dL) (
Table 1). Chest and abdominal radiography showed no definite abnormality. Acute appendicitis could not be totally ruled out; therefore, abdominal computed tomography (CT) was performed. It revealed an enlarged LN (1.9 cm) with suspicious internal necrosis and additional LN enlargement in the RLQ of the mesentery without demonstrable abnormal bowel wall thickening, including the appendix (
Fig. 1).
Fig. 1
Abdominal computed tomography. The largest lymph node is marked with red arrows.
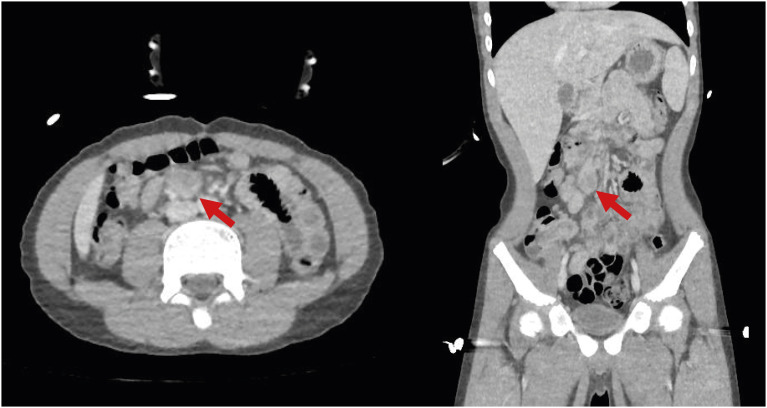

Table 1
Patient laboratory values during admission and at the outpatient clinic
|
Parameters |
HD#1 |
HD#3 |
HD#5 |
HD#8 |
OPD |
|
Hemoglobin, g/dL |
14.1 |
12.2 |
10.5 |
11.1 |
11.2 |
|
WBC, × 103/uL |
9.55 |
8.70 |
4.35 |
4.95 |
4.16 |
|
Neutrophil, % |
82.8 |
92.2 |
60.4 |
30.1 |
13.7 |
|
Lymphocytes, % |
10.4 |
4.7 |
30.4 |
51.0 |
74.1 |
|
Monocytes, % |
6.2 |
2.2 |
4.7 |
14.9 |
9.3 |
|
Platelets, × 103/uL |
167 |
123 |
174 |
432 |
423 |
|
Albumin, g/dL |
4.7 |
3.2 |
2.4 |
2.9 |
4.2 |
|
AST, IU/L |
38 |
24 |
31 |
35 |
69.0 |
|
ALT, IU/L |
21 |
18 |
12 |
17 |
52.0 |
|
Amylase, U/L |
18 |
15 |
ND |
ND |
ND |
|
Bilirubin, total, mg/dL |
0.68 |
0.45 |
0.26 |
0.43 |
0.60 |
|
LDH, U/L |
ND |
ND |
421 |
546 |
522 |
|
Creatinine, mg/dL |
0.59 |
0.66 |
0.46 |
0.42 |
0.47 |
|
hs-cardiac troponin T, ng/L |
ND |
13 |
ND |
ND |
ND |
|
Prothrombin time, sec |
ND |
13.5 |
ND |
13.3 |
ND |
|
INR |
ND |
1.15 |
ND |
1.13 |
ND |
|
aPPT, sec |
ND |
31.0 |
ND |
25.9 |
ND |
|
Fibrinogen, mg/dL |
ND |
442.9 |
ND |
ND |
ND |
|
D-dimer, ug/mL |
ND |
2.50 |
ND |
2.32 |
ND |
|
Ferritin, ng/mL |
ND |
2,485.0 |
ND |
616.21 |
542.30 |
|
ESR, mm/hr |
ND |
31 |
63 |
82 |
ND |
|
hs-C-reactive protein, mg/dL |
8.48 |
10.36 |
11.54 |
3.85 |
0.44 |
|
Procalcitonin, ng/mL |
1.54 |
ND |
ND |
ND |
ND |
|
Urine WBC, /HPF |
0–1 |
ND |
ND |
ND |
ND |

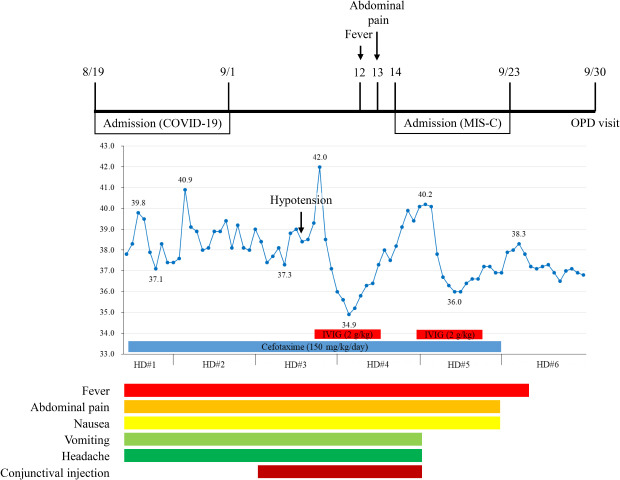
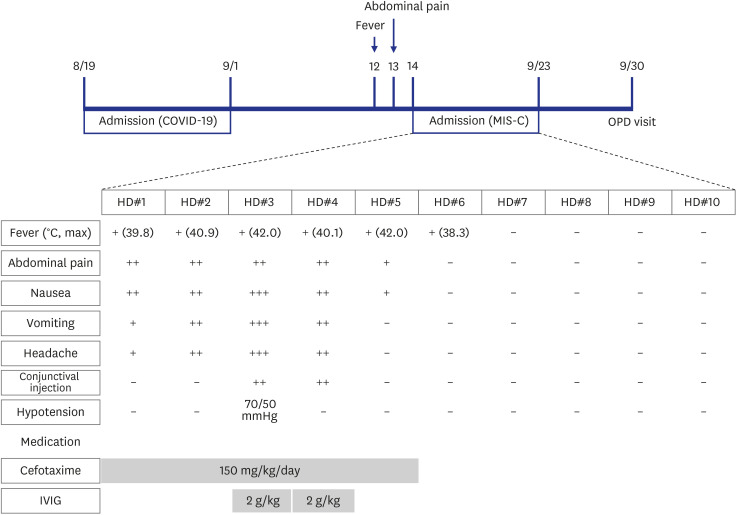
The patient was admitted for the management of possible mesenteric lymphadenitis and prescribed third-generation cephalosporin (cefotaxime). On hospital day (HD) 2, his chief complaints included headache, vomiting, and diarrhea. However, he was alert without notable signs of meningeal irritation. His body temperature increased to 40.9°C and did not fall below 38.0°C on that day. On HD3, conjunctival injection was noticed, as were complaints of myalgia and throat pain. His blood pressure abruptly dropped to 70/50 mmHg (pulse rate, 110 beats/min), and 500 mL of normal saline was administered intravenously. Echocardiography was performed to rule out cardiogenic shock, including left ventricle dysfunction, and showed only mild mitral valve regurgitation (MVR) without cardiac dysfunction or pericardial effusion. After 1.5 hours, his blood pressure was restored to 92/50 mmHg, without the use of inotropes. At night, his body temperature rose to 42.0°C, and the patient complained of whole-body pain. His unusually high body temperature, coupled with multiple organ (gastrointestinal; cardiac: MVR and hypotension; and bone marrow: anemia, neutrophilia, and thrombocytopenia) involvement with no other possible explanation, suggested MIS-C. Immunoglobulin (IVIG; 2 g/kg) was administered intravenously (
Fig. 2). On HD4, his body temperature dropped to 34.9°C, and his subjective condition markedly improved with frequent voiding. However, as time passed, his body temperature gradually increased to 40.1°C. He complained of generalized myalgia, and shivering was noticed. He was given oral naproxen (250 mg) but had no response. This prompted the administration of a second dose of IVIG (2 g/kg). On HD5, his body temperature dropped to 36.0°C with markedly improved general condition. On HD6, his body temperature rose to 38.3°C; however, it responded to antipyretics. Thereafter, his condition improved and body temperature remained stable. He was free from gastrointestinal and all other symptoms, including headache and conjunctival injection. The patient was discharged on HD10, delayed, in part, at the request of his caregiver. The patient visited the outpatient department a week after discharge without any symptoms or abnormal physical findings. Follow-up echocardiography did not show any sequelae in association with MIS-C.
Fig. 2
Timeline from the diagnosis of COVID-19 to the complete treatment of MIS-C.
COVID-19 = coronavirus disease 2019, MIS-C = multisystem inflammatory syndrome in children, OPD = outpatient department, HD = hospital day, IVIG = intravenous immunoglobulin.

The plaque reduction neutralization test performed on the serum prior to IVIG administration was conducted by the Korea Disease Control and Prevention Agency and yielded a result of 1:142 (positive ratio ≥ 1:10). Other tests were performed to rule out plausible alternative diagnoses (
Supplementary Table 1).
Important lessons were learned from the diagnosis and management of this patient. First, the importance of making local clinicians and pediatric gastroenterologists aware of the possibility of MIS-C during differential diagnosis, as they may be the first healthcare providers (HCPs) in cases of MIS-C following COVID-19. In this case, the patient presented with gastrointestinal symptoms; therefore, he was admitted under a pediatric gastroenterologist despite his history of COVID-19. There are relatively few mentions of pediatric gastroenterologists in relation to the treatment of MIS-C,
78 although the most prevalently involved organ system in MIS-C is the gastrointestinal system (92%).
4 Therefore, the role of pediatric gastroenterologists could be crucial in aiding the diagnosis of MIS-C, even though their role may be limited in the management of MIS-C.
The Korean Society of Pediatric Infectious Diseases (KSPID) has been actively monitoring the COVID-19 epidemic and maintains a website with up-to-date information, including COVID-19 pediatric guidelines and a chatroom for prompt and active communication. This communication tool has allowed for the widespread dissemination of epidemiologic and experiential information regarding the diagnosis and management of COVID-19 among leading pediatric infectious disease specialists throughout Korea. Insightful isolation practices were discussed, and the possibility of MIS-C was raised by one of the senior members of KSPID in the chatroom. Had it not been for her expertise, we believe that the patient's diagnosis could have been delayed, resulting in unknown consequences. Therefore, the second lesson we learned was the invaluable role of relevant discussions and collaborations among clinicians of diverse backgrounds, especially when coping with an unprecedented disease.
The benefits of immunomodulatory therapy have been suggested in both MIS-C and severe pediatric COVID-19 patients, even though no guidelines support the use of one immunomodulatory therapy over another.
910 Although IVIG is known to have adverse effects, as the patient satisfied the diagnostic criteria of MIS-C, the use of IVIG was justified.
11 IVIG dropped his body temperature to 34.9°C and greatly improved the patient's subjective condition. However, when his fever gradually worsened the following day, his condition became increasingly aggravated. Even though corticosteroids and anakinra are recommended when first-line treatment fails, we decided to continue with the treatment that showed clinical improvement in the patient. As there was no guidance regarding the interval between doses of IVIG in MIS-C, we decided to refer to the KD guidelines, which require a 48-hour interval between the first and second doses of IVIG
12; however, based on the patient's condition, we had to determine if we should wait an additional 24 hours before administering the second dose. Given his clinical improvement to the first dose of IVIG, we decided to administer the second dose outside the KD guidelines. The treatment was effective, and his general condition improved subjectively and objectively. The third lesson we learned was that following up-to-date recommendations for MIS-C treatment was important while actively exploring additional medications based on the patient's clinical response and the risks and benefits of each treatment.
Before his visit to CBNUH, the patient was tested for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) because of fever, and results were positive. However, since he had been previously diagnosed with COVID-19, viral detection could last without true infectivity.
13 Therefore, the patient was placed in the pediatric zone in the ED. HCPs quickly determined that they would not provide further medical care owing to the fear of infection. Although the evidence-based data regarding viral detection without infectivity was explained to the HCPs, they still remained hesitant. Eventually, the patient was moved to an isolation unit within the ED. In addition, a negative pressure isolation stretcher (implemented at CBNUH early in the course of the pandemic) was utilized to protect HCPs during the CT scan.
14 Therefore, the fourth lesson we learned was that the concerns of HCPs caring for patients with possible SARS-CoV-2 infectivity cannot be ignored and should be respected.
15
This 12-year-old boy was one of the first two cases of MIS-C in Korea. The clinical aspects of this case distinctly differed from the other case. The proper diagnosis of MIS-C was challenging, and the case provided considerable lessons for attending physicians that would help others who may encounter similar patients. Above all, disease awareness was essential to the proper diagnosis and management of MIS-C.
Ethics statement
This study was approved by the Institutional Review Bboard (IRB) of Chungbuk National University Hospital (IRB No. 2020-09-016). Informed written consent for the publication of this report was obtained from the mother of the patient.