INTRODUCTION
The coronavirus disease 2019 (COVID-19) outbreak which was caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is a global pandemic infectious disease. Daegu City, Korea, had the first large outbreak of COVID-19 outside China.
1 In Daegu, the outbreak crisis started on February 18, 2020, and peaked on February 29, 2020, with 741 new cases confirmed in a day.
23 This crisis was caused by Shincheonji Daegu Church worship events. The outbreak was controlled by the active management of patients with isolation in hospitals and 15 therapeutic living centers, treatment of patients and prevention with civic participation in the use of mask, hand hygiene, and social distancing.
3 Despite immense pressures on its health-care system, Daegu was able to avoid the destruction of the health-care system with these interventions that relieved hospital shortages and protected health-care workers.
1 After the outbreak, the detection rate of confirmed patients was lower than other areas.
A report about the clinical course and outcomes of COVID-19 in Korea with 3,060 patients, gave us a lot of information for the COVID-19 patients.
4 A report that focused on the test, tracing and treatment of COVID-19 patients in Seoul was also published.
5 However, whole picture of Daegu COVID-19 outbreak, which is the first outbreak crisis in Korea, was not reported until now. Therefore, the aim of this study is to evaluate and describe the clinical characteristics, risk factors for the case fatality rate, and duration of isolation in patients with COVID-19 in Daegu Metropolitan City (February to June 2020) after making and clearing of Daegu COVID-19 cohort data.
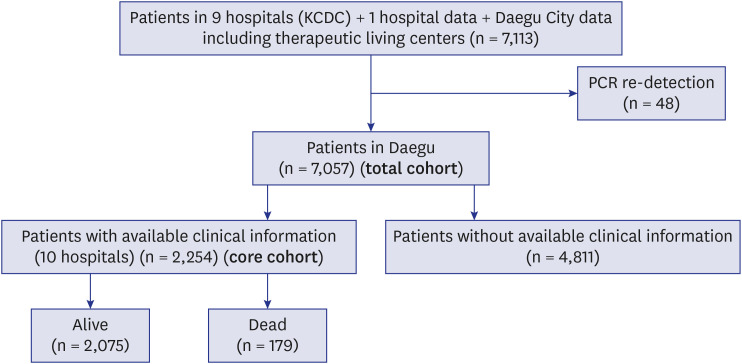
In this study, the details of all patients registered in Daegu Metropolitan City with polymerase chain reaction (PCR)-confirmed COVID-19 are presented. Only a few city-wide large-scale cohort studies on patients with COVID-19 were conducted.
DISCUSSION
This retrospective cohort study showed the clinical manifestations in the Daegu City COVID-19 outbreak and identified the distributions of the severity of patients with COVID-19 in the epidemic of a metropolitan city. The isolation methods in the outbreak were also shown. Moreover, this study found several risk factors for death in adults hospitalized with COVID-19. The risk factors for sustained viral detection in the respiratory tract included severe disease and old age.
Facility isolation (therapeutic living center) was first introduced in Korea during the Daegu COVID-19 outbreak.
3 This isolation method was effective for resolving hospital-bed shortage.
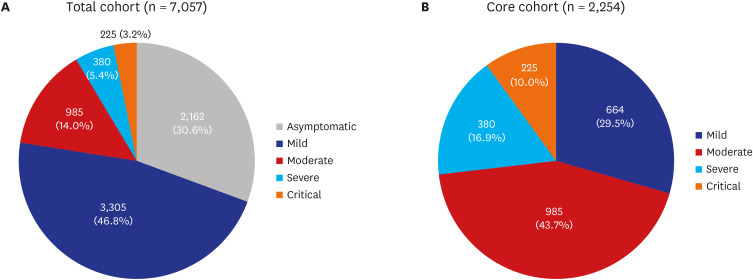
3 Facility isolation for asymptomatic and mild cases were crucial to helping overcome COVID-19 outbreaks in Daegu, Korea because > 80% of cases did not require special therapies, such as oxygen supplementation or parenteral fluid infusion or antiviral therapy.
6 This study revealed that approximately 40% of patients were isolated using this method.
The clinical spectrum of COVID-19 infection is wide, encompassing asymptomatic infection, mild upper respiratory tract illness, and severe viral pneumonia with respiratory failure and even death in the previous reports.
7 Previous studies reported that COVID-19 is characterized by various clinical manifestations.
8 In a typical case, a high fever appears after dry cough; in some cases, viral pneumonia develops and progresses, resulting in shortness of breath.
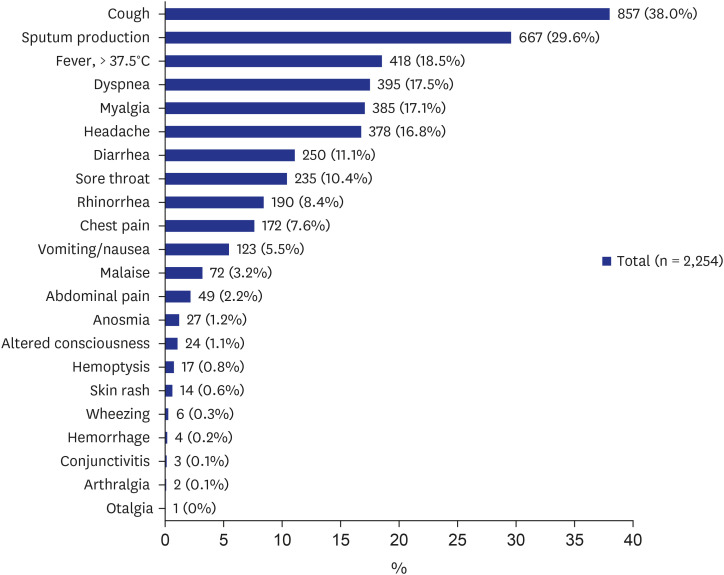
89 Common symptoms among patients with COVID-19 include fever, dry cough, shortness of breath (dyspnea), muscle ache (myalgia), confusion, headache, sore throat, rhinorrhea, chest pain, diarrhea, nausea/vomiting, conjunctival congestion, nasal congestion, sputum production, fatigue (malaise), hemoptysis, and chills.
810111213 Some published articles and a study from Daegu also reported the significance of anosmia or ageusia (loss of taste) as symptoms of COVID-19.
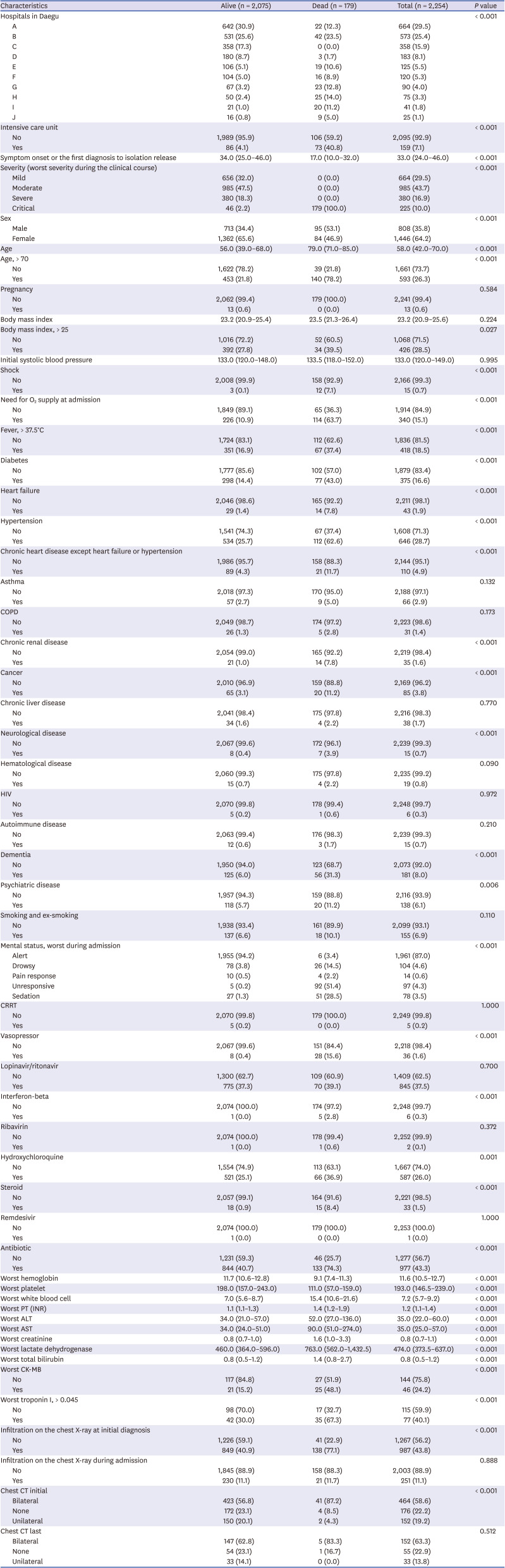
14151617 However, only 1.2% of the patients in the present study presented with anosmia, indicating that clinicians did not actively obtain the presence of anosmia in the early period of the outbreak. The clinical manifestations of the present study are similar to the previous reports characterized primarily by fever, cough, dyspnea, and bilateral infiltrates on chest imaging.
18
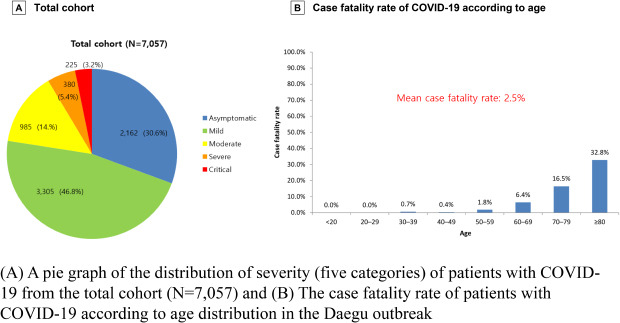
In the present study, asymptomatic patient proportion is approximately 30%. In some studies of asymptomatic individuals who were hospitalized and monitored, approximately 77% to 89% remained asymptomatic over time.
19 The definition of “asymptomatic” varies across studies, depending on which specific symptoms were assessed. A review paper estimated that it is as 40%–45% based on data from three large cohorts that identified cases through population-based testing.
20 However, in most studies, longitudinal follow-up to assess for symptom development was not performed.
19 Therefore, an overestimation of the number of asymptomatic COVID-19 patients might be possible. In the present study, “asymptomatic” means no symptom described or observed during the whole duration of infection, which can explain the lesser proportion of asymptomatic infection.
The case fatality rate of patients with COVID-19 varies among countries related to demographics, patient comorbidities, surge capacity of healthcare systems, and quality of medical care.
4 The case fatality rate of COVID-19 in the Daegu City outbreak was 2.5%, which is higher than the recently reported estimation of case fatality (0.68%) in a meta-analysis report.
21 Also, a paper reported that the infection fatality rate for Spain as a whole was 1.15% (range, 0.13% to 3.25%) and is not fixed.
22 The Spanish regions with more rapid and extensive spread of SARS-CoV-2 had higher case fatality rate.
22 In the outbreak in China, it was reported that the case fatality rate was 2.3% (1,023 of 44,672 confirmed cases), which is similar to that of the present study.
23
A study with 3,060 Korean COVID-19 patients reported that the mortality was 1.1% (27/2,524).
4 The differences in mortality among study populations may be explained by the differences that include age distribution, the prevalence of comorbid conditions of the patients, and public health crisis preparedness and response of health-care systems. The median age of our study population was 58 years, which is older than the median (43 years) of the study population with 3,060 Korean COVID-19 patients.
4 Higher mortality in our study may be related to this different age distribution.
The Daegu City outbreak was the first one in Korea. Unpreparedness to the explosive COVID-19 outbreak at spring in 2020 might have contributed to the higher mortality rate in Daegu. Moreover, hospital shortage in the early period of the outbreak was the most prominent obstacle in overcoming the outbreak.
3 A telephone severity scoring system and therapeutic living centers solved acute hospital-bed shortage during the COVID-19 outbreak in Daegu, Korea.
3
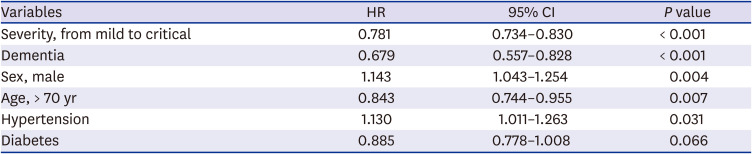
Previously, older age has been reported as an important independent predictor of mortality in COVID-19.
724 This study also found that increased age (age > 70) was associated with death in patients with COVID-19.
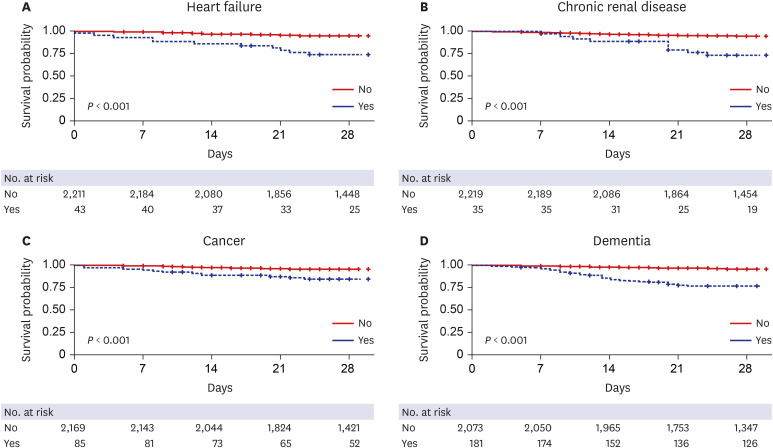
Cardiovascular disease, diabetes, respiratory disease (including severe asthma), obesity, a history of hematological malignancy or recent other cancers, kidney, liver and neurological diseases, and autoimmune conditions were reported to be risk factors for mortality.
24 A meta-analysis with 44,672 patients with COVID-19 reported that cardiovascular disease, hypertension, diabetes, respiratory disease, and cancers were the risk factors for fatality.
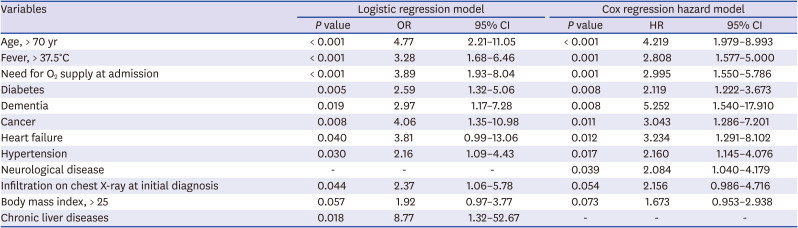
25 We found that age > 70, fever (> 37.5°C), need for O
2 supply at admission, diabetes, dementia, cancer, heart failure, hypertension, and neurological disease were independent risk factors for 28-day mortality. This finding is similar to that of previous reports on the most important risk factors. The present study emphasizes the significance of dementia and neurological disease as risk factors of mortality. Dementia as a predictor of mortality of COVID-19 patients is also reported in some studies.
2627
A study with COVID-19 with Daegu population was published and reported that mortality as 2.6% (18/694) and significant risk factors for severe disease were lymphopenia, lactate dehydrogenase, C-reactive protein, and low albumin in the study.
28 However, the study was conducted with univariate analysis.
28 A study with COVID-19 with 352 Korean patients (hospitalized in Dongguk University Gyeongju Hospital and the Andong Medical Center) with multi-variable regression analysis reported age (≥ 70 years), a history of malignancy, fever (≥ 37.5°C), and diabetes were significant risk factors of mortality.
27 We found that the risk factors for 28-day case fatality rate with multi-variable logistic analysis and Cox hazard model with a relatively large population (2,254 patients).
Comparison between COVID-19 studies had limitations because the collected variables and definition of severity category are largely different.
The duration of virus detection determined the isolation period during the study period. Two consecutive negative COVID-19 PCR tests with a 24-hour interval were required for the release from isolation in Daegu, Korea. Median (33 days) of isolation duration was over a month; this long isolation occupied the beds or isolation facilities and made medical resource shortage. This criteria for the release from isolation were used among the patients in this study population. Recently, Korea Disease Control and Prevention Agency revised the criteria of stopping isolation to avoid medical resource shortage. The modified criteria for discharge include no 3-day symptoms and passing 10 days after the first symptom onset. This modification was based on scientific evidence of a very low possibility of virus transmission after 10 days from the onset of infection.
29
The present study has some limitations. First, due to the retrospective study design, not all information is available. Therefore, we focused on the initial and worst data for clinical information. Moreover, the laboratory values were not included such as C-reactive protein and procalcitonin and D-dimer for the analysis of risk for mortality due to inconsistent values in the multicenter study. Instead, initial clinical manifestations were mainly used for predicting outcomes. Second, the retrospective study design also has difficulty in the assessment of the efficacy of treatment such as steroids, hydroxychlorquine, and anti-retroviral agents such as lopinavir/ritonavir.
In conclusion, this study described the clinical manifestations in the city-wide outbreak cohort of COVID-19 patients in Daegu City, Korea. The case fatality rate was 2.5% in the first outbreak in Korea in 2020. Asymptomatic to mild patients were approximately 77% of the total cohort (asymptomatic: 30.6%). Risk factors, including older age, need for O2 supply, dementia, and neurological disorder at admission could help clinicians identify patients with poor prognosis at an early stage.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download