This article has been
cited by other articles in ScienceCentral.
Abstract
Background
The quarantine process at a country's port of entry has an important role in preventing an influx of coronavirus disease 2019 (COVID-19) cases from abroad and further minimizing the national healthcare burden of COVID-19. However, there has been little published on the process of COVID-19 screening among travelers entering into a country. Identifying the characteristics of COVID-19 infected travelers could help attenuate the further spread of the disease.
Methods
The authors analyzed epidemiological investigation forms and real-time polymerase chain reaction (PCR) results for severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) of entrants to Incheon International Airport between March 11 to April 30, 2020. We performed univariate and multivariate logistic regression analysis to determine the odds of positive SARS-CoV-2 result.
Results
A total of 11,074 entrants underwent reverse-transcription PCR for SARS-CoV-2, resulting 388 confirmed cases of COVID-19 infection. COVID-19 had a strong association with the reported loss of smell or taste and association with self-reported fever, chill, cough, and vomiting. If a traveler reported contact with an individual with either respiratory symptoms or confirmed COVID-19 in the last two weeks directly prior to landing, the probability of a positive result was increased.
Conclusion
If overseas travelers experience loss of smell or taste in the two weeks prior to arrival, they may require an immediate examination to rule out COVID-19 at a port of entry. As to measure body temperature upon arrival at a port of entry, it is important to screen for any occurrence of fever within the two weeks prior to travel. Also, information with epidemiological relevance, such as recent contact with an individual suffering from any respiratory symptoms or with confirmed COVID-19, should be included in COVID-19 screening questionnaires for international travelers.
Go to :

Graphical Abstract
Go to :

Keywords: COVID-19, Coronavirus, Anosmia, Ageusia, Quarantine, Travel
INTRODUCTION
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is highly infectious and R
O as high as 2.3 is reported.
1 As of December 9, 2020, there are about 600,000 newly confirmed cases a day worldwide, and the number does not seem to be attenuating. In the earlier stages of the coronavirus disease 2019 (COVID-19) pandemic, Korea was one of the countries with the most confirmed cases, but is now ranked around ninetieth among countries with the most confirmed cases.
2 While many countries have strategically blocked their borders completely or partially, the Korean borders have remained open to foreign nationals and citizens alike.
To minimize the domestic spread of the disease, strict screening of symptomatic travelers for COVID-19 is conducted at ports of entry into Korea. Several thousand health screening records of travelers with various symptoms, and the results of polymerase chain reaction (PCR) for COVID-19 are documented every month. So far, there has been little published on traveler screening for COVID-19 during international travel. Identifying the characteristics of COVID-19 among infected travelers could help to attenuate the spread of the disease between countries.
Go to :

METHODS
Screening process
All incoming travelers to the Incheon International Airport, Korea are required to fill out health questionnaires (
Fig. 1).
3 Travelers were also required to report an administration of a fever-subsiding drug and contact with an individual either suffering from respiratory symptoms or with confirmed COVID-19. If a traveler reports a symptom, a drug administration or contact with the patients, the traveler is classified as a screening target. At the same time, screening based on body temperature measurement by the infrared camera is performed with the threshold of 37.5°C or higher.
 | Fig. 1Health questionnaire.
|
The screening target undergoes an interview by a physician and body temperature is measured again through the tympanic membrane. Based on the questionnaire and the interview, the physician writes an epidemiological investigation form. The interviewing physician determines whether to perform SARS-CoV-2 PCR on a case-by-case basis, considering history (medical, working or travel), current clinical condition, the recent epidemiologic status of the country of departure, etc. If required, nasopharyngeal and throat swabs for SARS-CoV-2 PCR is performed at a designated site out of the terminal building.
Laboratory confirmation
Laboratory confirmation of SARS-CoV-2 was performed at the Laboratory Department of Incheon Airport National Quarantine Station. The reverse-transcription PCR assay was conducted. Extraction of nucleic acids from the samples was performed with the commercialized nucleic acid extraction kits following; QIAamp Viral RNA Mini kit (QIAGEN Inc., Hilden, Germany), Maxwell RSC Viral Total Nucleic Acid Purification Kit (Promega, Madison, WI, USA), MagNa Pure LC/Total Nucleic Acid Isolation Kit (Roche Molecular Systems Inc., Penzberg, Germany). After extraction, amplifications were done with PowerChek™ PCR kit (KogeneBiotech, Seoul, Korea) and the Applied Biosystems 7500 Fast Real-Time PCR System (ThermoFisher Scientific, Waltham, MA, USA). Test results were reported as qualitatively positive or negative for the presence of nucleic acid from SARS-CoV-2.
Data collection
Information of travelers was collected using an epidemiological investigation form. Data recorded from March 11 to April 30, 2020 were included in the study (
Fig. 2). Researchers read each epidemiological investigation form, and variables were coded manually using Microsoft Excel. Variables were age, sex, tympanic temperature, clinical symptoms, epidemiological relevance, and administration of a fever-subsiding drug (NSAIDs, paracetamol, steroid, etc.; identified during the interview) in the twelve hours prior to landing.
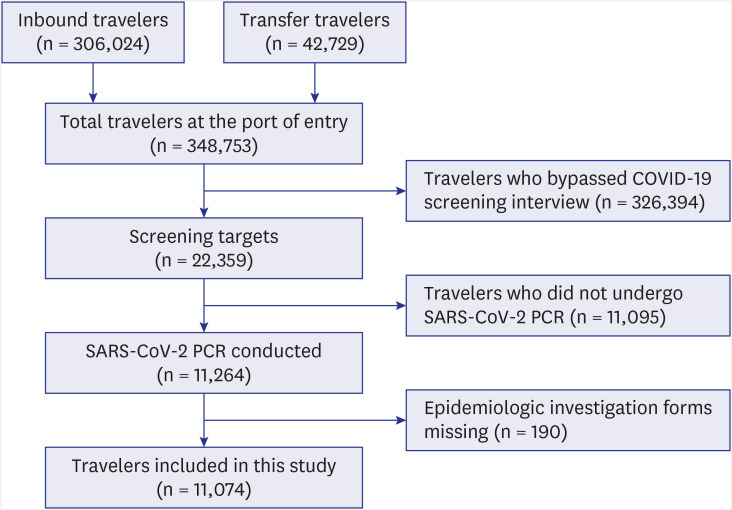 | Fig. 2
Flow diagram of case inclusion process.
COVID-19 = coronavirus disease 2019, SARS-CoV-2 = severe acute respiratory syndrome coronavirus-2, PCR = polymerase chain reaction.

|
Clinical symptoms were mainly those listed on the health questionnaire; fever, chill, myalgia, cough, sputum, shortness of breath, sore throat, rhinorrhea/nasal congestion, headache, abdominal pain, diarrhea, and vomiting. Loss of smell or taste was included because it is frequently described in epidemiological investigation forms by physicians. Epidemiological relevance was contact with an individual suffering from any respiratory symptoms or with confirmed COVID-19.
Travelers were classified into SARS-CoV-2 positive or SARS-CoV-2 negative groups based on PCR results.
Statistical analysis
IBM SPSS Statistics for Windows version 25.0 (IBM Corp, Armonk, NY, USA) was used for comparison of demographic data and χ2 test. RStudio version 1.3 (RStudio Team, Boston, MA, USA) was used for logistic regression analysis and graph plotting. Categorical data were analyzed using the χ2 test. Univariate and multivariate logistic regression analysis was performed to determine the odds ratio (OR) and 95% confidence interval (CI) for OR of positive SARS-CoV-2 result by the variables. A probability value of < 0.05 was regarded as statistically significant.
Ethics statement
The protocol of the current study was reviewed and approved by the Institutional Review Board of Korea Disease Control and Prevention Agency (approval No.2020-09-01-P-A). Because of the retrospective and non-invasive study design, the requirement for informed consent was waived by the board.
Go to :

RESULTS
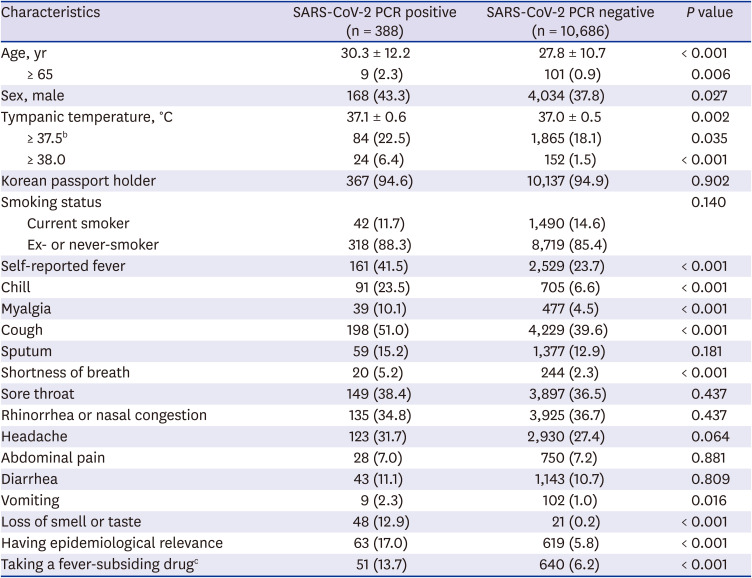
PCR was performed on samples from 11,074 travelers and 388 (3.5%) were determined to be SARS-CoV-2 positive based on PCR results (
Table 1). The majority of travelers (94.9%) were Korean citizens. The mean age of the travelers was about 27.9 ± 10.8 years old and 4,202 of 11,074 travelers (37.9%) were male. The mean tympanic temperature at the port of entry was 37.0°C ± 0.5°C.
Table 1
Characteristics and reported symtpomsa of screening targets (n = 11,074)
|
Characteristics |
SARS-CoV-2 PCR positive (n = 388) |
SARS-CoV-2 PCR negative (n = 10,686) |
P value |
|
Age, yr |
30.3 ± 12.2 |
27.8 ± 10.7 |
< 0.001 |
|
≥ 65 |
9 (2.3) |
101 (0.9) |
0.006 |
|
Sex, male |
168 (43.3) |
4,034 (37.8) |
0.027 |
|
Tympanic temperature, °C |
37.1 ± 0.6 |
37.0 ± 0.5 |
0.002 |
|
≥ 37.5b
|
84 (22.5) |
1,865 (18.1) |
0.035 |
|
≥ 38.0 |
24 (6.4) |
152 (1.5) |
< 0.001 |
|
Korean passport holder |
367 (94.6) |
10,137 (94.9) |
0.902 |
|
Smoking status |
|
|
0.140 |
|
Current smoker |
42 (11.7) |
1,490 (14.6) |
|
Ex- or never-smoker |
318 (88.3) |
8,719 (85.4) |
|
Self-reported fever |
161 (41.5) |
2,529 (23.7) |
< 0.001 |
|
Chill |
91 (23.5) |
705 (6.6) |
< 0.001 |
|
Myalgia |
39 (10.1) |
477 (4.5) |
< 0.001 |
|
Cough |
198 (51.0) |
4,229 (39.6) |
< 0.001 |
|
Sputum |
59 (15.2) |
1,377 (12.9) |
0.181 |
|
Shortness of breath |
20 (5.2) |
244 (2.3) |
< 0.001 |
|
Sore throat |
149 (38.4) |
3,897 (36.5) |
0.437 |
|
Rhinorrhea or nasal congestion |
135 (34.8) |
3,925 (36.7) |
0.437 |
|
Headache |
123 (31.7) |
2,930 (27.4) |
0.064 |
|
Abdominal pain |
28 (7.0) |
750 (7.2) |
0.881 |
|
Diarrhea |
43 (11.1) |
1,143 (10.7) |
0.809 |
|
Vomiting |
9 (2.3) |
102 (1.0) |
0.016 |
|
Loss of smell or taste |
48 (12.9) |
21 (0.2) |
< 0.001 |
|
Having epidemiological relevance |
63 (17.0) |
619 (5.8) |
< 0.001 |
|
Taking a fever-subsiding drugc
|
51 (13.7) |
640 (6.2) |
< 0.001 |

The mean age was 27.8 ± 10.7 years old in the SARS-CoV-2 negative and 30.3 ± 12.2 years old in the SARS-CoV-2 positive (median age, 25 and 26 years, respectively) (
Fig. 3). Mean tympanic temperature was 37.0°C ± 0.5°C in the SARS-CoV-2 negative and 37.1°C ± 0.6°C in the SARS-CoV-2 positive (median temperature, 37.0°C and 37.1°C; interquartile range, 36.7°C–37.3°C and 36.7°C–37.4°C, respectively) (
Fig. 4). The proportions of Korean citizens and current smokers were similar in the two groups.
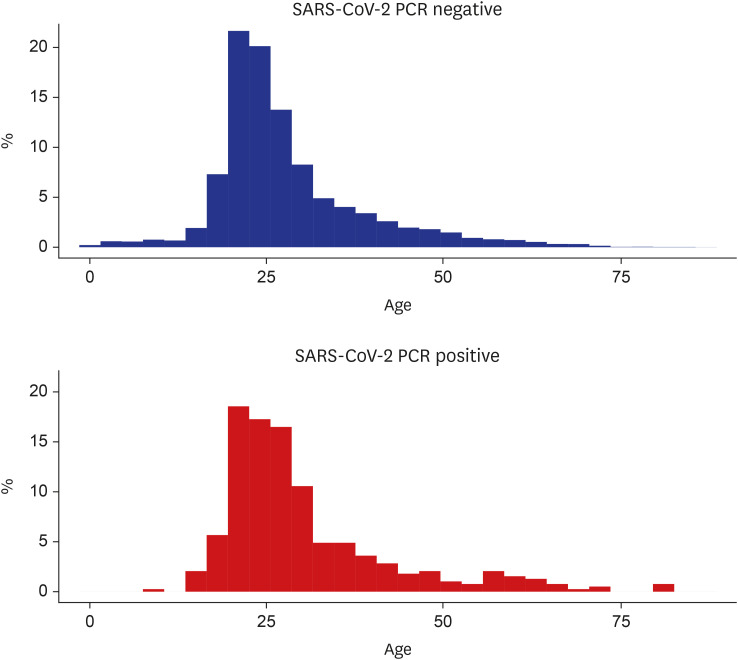 | Fig. 3
Histogram of age at the port of entry in each group.
SARS-CoV-2 = severe acute respiratory syndrome coronavirus-2, PCR = polymerase chain reaction.

|
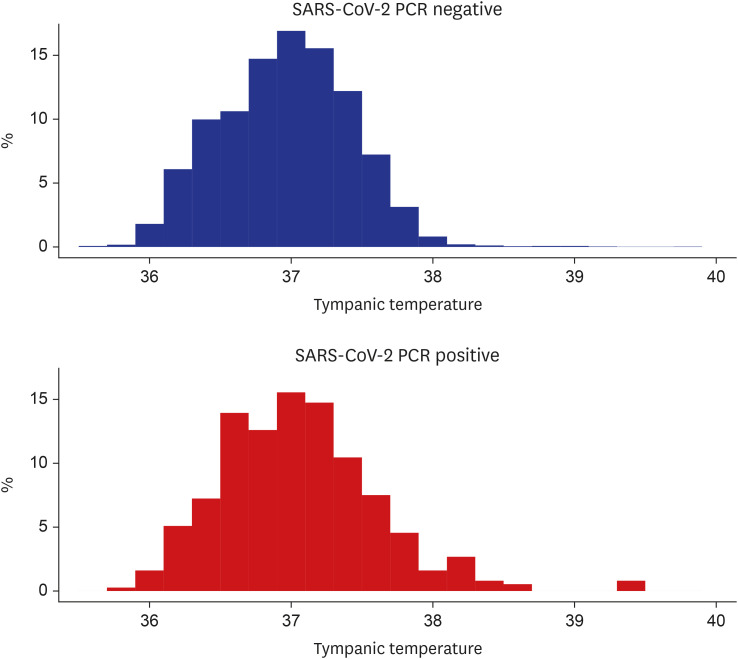 | Fig. 4
Histogram of tympanic temperature at the port of entry in each group.
SARS-CoV-2 = severe acute respiratory syndrome coronavirus-2, PCR = polymerase chain reaction.

|
Self-reported fever, chill, myalgia, cough, shortness of breath, vomiting and loss of smell or taste were symptoms reported significantly more frequently in the SARS-CoV-2 positive than in the SARS-CoV-2 negative. The most common symptom reported in SARS-CoV-2 positive travelers was cough (198 of 388, 51.0%), followed by fever (161 of 388, 41.5%). Epidemiologic relevance was reported more frequently in the SARS-CoV-2 positive than in the SARS-CoV-2 positive. Likewise, administration of fever-subsiding drugs was more common in the SARS-CoV-2 positive.
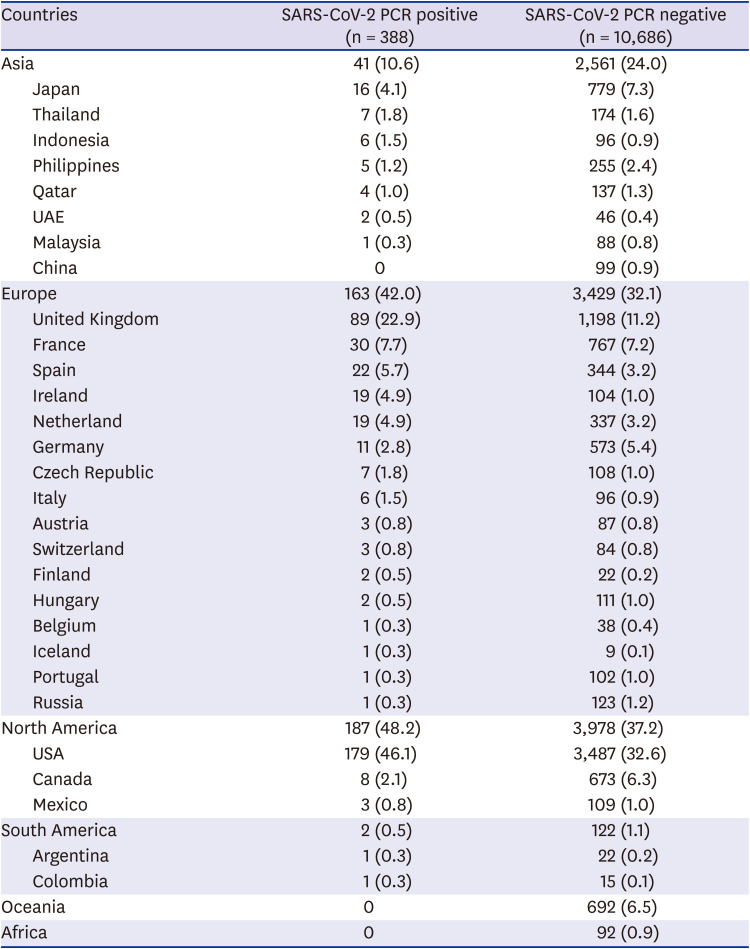
The country which the most screening targets visited was the United States, followed by the United Kingdom (
Table 2). The most diverse countries in Europe have been visited by travelers. Travelers confirmed with COVID-19 had not visited countries of Oceania and Africa two weeks prior to arrival.
Table 2
The countries of visit in the two weeks prior to arrival in screening targetsa
|
Countries |
SARS-CoV-2 PCR positive (n = 388) |
SARS-CoV-2 PCR negative (n = 10,686) |
|
Asia |
41 (10.6) |
2,561 (24.0) |
|
Japan |
16 (4.1) |
779 (7.3) |
|
Thailand |
7 (1.8) |
174 (1.6) |
|
Indonesia |
6 (1.5) |
96 (0.9) |
|
Philippines |
5 (1.2) |
255 (2.4) |
|
Qatar |
4 (1.0) |
137 (1.3) |
|
UAE |
2 (0.5) |
46 (0.4) |
|
Malaysia |
1 (0.3) |
88 (0.8) |
|
China |
0 |
99 (0.9) |
|
Europe |
163 (42.0) |
3,429 (32.1) |
|
United Kingdom |
89 (22.9) |
1,198 (11.2) |
|
France |
30 (7.7) |
767 (7.2) |
|
Spain |
22 (5.7) |
344 (3.2) |
|
Ireland |
19 (4.9) |
104 (1.0) |
|
Netherland |
19 (4.9) |
337 (3.2) |
|
Germany |
11 (2.8) |
573 (5.4) |
|
Czech Republic |
7 (1.8) |
108 (1.0) |
|
Italy |
6 (1.5) |
96 (0.9) |
|
Austria |
3 (0.8) |
87 (0.8) |
|
Switzerland |
3 (0.8) |
84 (0.8) |
|
Finland |
2 (0.5) |
22 (0.2) |
|
Hungary |
2 (0.5) |
111 (1.0) |
|
Belgium |
1 (0.3) |
38 (0.4) |
|
Iceland |
1 (0.3) |
9 (0.1) |
|
Portugal |
1 (0.3) |
102 (1.0) |
|
Russia |
1 (0.3) |
123 (1.2) |
|
North America |
187 (48.2) |
3,978 (37.2) |
|
USA |
179 (46.1) |
3,487 (32.6) |
|
Canada |
8 (2.1) |
673 (6.3) |
|
Mexico |
3 (0.8) |
109 (1.0) |
|
South America |
2 (0.5) |
122 (1.1) |
|
Argentina |
1 (0.3) |
22 (0.2) |
|
Colombia |
1 (0.3) |
15 (0.1) |
|
Oceania |
0 |
692 (6.5) |
|
Africa |
0 |
92 (0.9) |

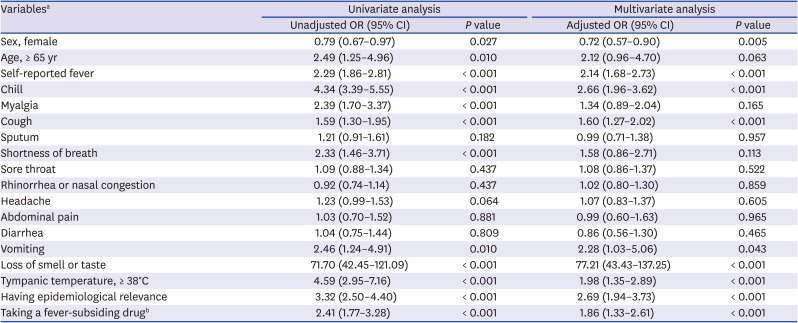
Multivariate logistic regression analysis indicated the following variables to be significantly associated with positive results of PCR for SARS-CoV-2: Male sex, self-reported fever, chill, cough, vomiting, loss of smell or taste, epidemiological relevance, and administration of any fever-subsiding drug in the twelve hours prior to arrival (
Table 3). Loss of smell or taste had the strongest association with positive results of PCR for SARS-CoV-2 (adjusted OR, 77.21; 95% CI, 43.43-137.25).
Table 3
Univariate and multivariate analysis for SARS-CoV-2 PCR positive result
|
Variablesa
|
Univariate analysis |
Multivariate analysis |
|
Unadjusted OR (95% CI) |
P value |
Adjusted OR (95% CI) |
P value |
|
Sex, female |
0.79 (0.67–0.97) |
0.027 |
0.72 (0.57–0.90) |
0.005 |
|
Age, ≥ 65 yr |
2.49 (1.25–4.96) |
0.010 |
2.12 (0.96–4.70) |
0.063 |
|
Self-reported fever |
2.29 (1.86–2.81) |
< 0.001 |
2.14 (1.68–2.73) |
< 0.001 |
|
Chill |
4.34 (3.39–5.55) |
< 0.001 |
2.66 (1.96–3.62) |
< 0.001 |
|
Myalgia |
2.39 (1.70–3.37) |
< 0.001 |
1.34 (0.89–2.04) |
0.165 |
|
Cough |
1.59 (1.30–1.95) |
< 0.001 |
1.60 (1.27–2.02) |
< 0.001 |
|
Sputum |
1.21 (0.91–1.61) |
0.182 |
0.99 (0.71–1.38) |
0.957 |
|
Shortness of breath |
2.33 (1.46–3.71) |
< 0.001 |
1.58 (0.86–2.71) |
0.113 |
|
Sore throat |
1.09 (0.88–1.34) |
0.437 |
1.08 (0.86–1.37) |
0.522 |
|
Rhinorrhea or nasal congestion |
0.92 (0.74–1.14) |
0.437 |
1.02 (0.80–1.30) |
0.859 |
|
Headache |
1.23 (0.99–1.53) |
0.064 |
1.07 (0.83–1.37) |
0.605 |
|
Abdominal pain |
1.03 (0.70–1.52) |
0.881 |
0.99 (0.60–1.63) |
0.965 |
|
Diarrhea |
1.04 (0.75–1.44) |
0.809 |
0.86 (0.56–1.30) |
0.465 |
|
Vomiting |
2.46 (1.24–4.91) |
0.010 |
2.28 (1.03–5.06) |
0.043 |
|
Loss of smell or taste |
71.70 (42.45–121.09) |
< 0.001 |
77.21 (43.43–137.25) |
< 0.001 |
|
Tympanic temperature, ≥ 38°C |
4.59 (2.95–7.16) |
< 0.001 |
1.98 (1.35–2.89) |
< 0.001 |
|
Having epidemiological relevance |
3.32 (2.50–4.40) |
< 0.001 |
2.69 (1.94–3.73) |
< 0.001 |
|
Taking a fever-subsiding drugb
|
2.41 (1.77–3.28) |
< 0.001 |
1.86 (1.33–2.61) |
< 0.001 |

Go to :

DISCUSSION
Our results indicated a strong association between COVID-19 and loss of smell or taste. The positive predictive value of loss of smell or taste was as high as 69.6%. This symptom also showed a strong association with COVID-19 in other studies, and it is still reported to be remarkable when adjusted for other symptoms or demographic factors such as sex and age.
4 When an incoming traveler reports loss of smell or taste in the two weeks prior to travel, an immediate diagnostic test may be appropriate for the prevention of the spread of the disease.
Fever is a very common symptom in COVID-19 patients. Guan et al.
5 report that 85% of patients with COVID-19 present with pyrexia during the course of their illness. In the current study, travelers who reported a fever had over two-fold increase in the probability of SARS-CoV-2 positive PCR results (adjusted OR, 2.14; 95% CI, 1.68–2.73). However, it is important to note that pyrexia is not always present throughout the COVID-19 disease course.
1 Among the 388 travelers with PCR-confirmed COVID-19 included in the current study, only twenty-four travelers (6.4%) had a tympanic temperature of 38.0°C or higher at the port of entry. Considering this, we believe it is just important to screen for any occurrence of fever within the two weeks prior to travel, as it is to measure body temperature upon arrival at a port of entry.
About half of the SARS-CoV-2 positive group (51.0%) reported cough symptoms in the two weeks prior to travel. Cough is the most common respiratory symptom associated with COVID-19, with more than half of COVID-19 patients reported to suffer from cough symptoms.
167 In a study conducted on health care workers by Clemency et al.,
7 dry cough was the symptom with the highest sensitivity (74%). In the current study, chill symptoms were seldom reported in the SARS-CoV-2 negative travelers, but were reported in about a quarter (23.5%) of the SARS-CoV-2 positive travelers. Adjusted with other variables, chill had an OR of 2.66 (95% CI, 1.96–3.62). Therefore, inquiring about chill symptoms may also be of remarkable value when screening travelers.
We noted that males were more likely to be confirmed with COVID-19 infection, which is frequently reported elsewhere.
689 While some studies indicate that male sex is also associated with severe disease course and death,
610 severe cases were rare and poorly documented among the travelers included in the current study, making them impossible to be included in the current analysis. Other studies report old age to be as another predominant factor of COVID-19 infection.
8 In our study, COVID-19 confirmed cases tend to be more common in the elderly; however, there was no statistical significance in multivariate logistic regression analysis.
One limitation of our study is that not all travelers entering into Korea were included in the control group. If a passenger intentionally did not report symptoms and body temperature was normal, the passenger could bypass screening. Also, asymptomatic patients could not be included in our study. Thus, the characteristics described here are only applicable for travelers selected as screening targets. However, this study identified common factors associated with COVID-19 infection that could be screened for, even in the general population.
Further study should be conducted among all incoming travelers. Measures to predict asymptomatic cases is needed to prevent COVID-19 spread because asymptomatic cases are frequent on the course of the disease.
11 Combining the result of this study with the measures to identify asymptomatic patients would greatly facilitate quarantine processes around the world and attenuate the further spread of COVID-19.
Go to :








 PDF
PDF Citation
Citation Print
Print







 XML Download
XML Download