Abstract
The cause of epithelioid granulomatous inflammation varies widely depending on the affected organ, geographic region, and whether the granulomas morphologically contain necrosis. Compared with other organs, the etiological distribution and morphological patterns of pleural epithelioid granulomas have rarely been investigated. We evaluated the final etiologies and morphological patterns of pleural epithelioid granulomatous inflammation in a tuberculosis (TB)-prevalent country. Of 83 patients with pleural granulomas, 50 (60.2%) had confirmed TB pleurisy (TB-P) and 29 (34.9%) had probable TB-P. Four patients (4.8%) with non-TB-P were diagnosed. With the exception of microbiological results, there was no significant difference in clinical characteristics and granuloma patterns between the confirmed TB-P and non-TB-P groups, or between patients with confirmed and probable TB-Ps. These findings suggest that most pleural granulomatous inflammation (95.2%) was attributable to TB-P in TB-endemic areas and that the granuloma patterns contributed little to the prediction of final diagnosis compared with other organs.
Graphical Abstract
Granulomatous inflammation is a major histological pattern produced in response to various injuries. The etiology of granulomatous inflammation varies widely depending on the affected organ, geographic region, and morphological patterns, such as necrotic or non-necrotic granuloma.12 In the organ involved, information regarding etiological distribution and morphological patterns can aid in predicting the etiology of a given granuloma.13 However, in contrast to lung, skin, and lymph node, there are few data regarding the etiological distribution and morphological pattern of epithelioid granulomatous inflammation in the pleura. In addition, although granulomatous pleural inflammations without a specific etiology identified have been considered and treated as tuberculous (TB) pleurisy (TB-P) in TB-endemic areas, their characteristics and treatment outcomes have rarely been investigated. Thus, this study evaluated the etiology and histological patterns of epithelioid granulomatous pleural inflammation in a TB-endemic area, and the characteristics and anti-TB treatment outcomes of patients with probable TB-P diagnosed by histological granuloma but no microbiological confirmation.
Patients who had epithelioid granulomatous inflammation on pleural biopsy at Kyungpook National University Hospital in Korea, a country with an intermediate TB burden,4 between January 2008 and March 2020 were included in this study. Pleural biopsy was indicated for the confirmation or exclusion of granulomatous pleural disease suspected in the patients with exudative pleural effusion. These patients were retrospectively reviewed and their final etiologies identified. Patients with TB-P were classified into 2 groups: confirmed and probable TB-P. The confirmed TB-P group included patients who had granulomatous pleural histology with positive culture or polymerase chain reaction (PCR) for Mycobacterium tuberculosis (MTB) in pleural fluid, pleural tissue, sputum, or bronchial aspirate. The probable TB-P group included patients who had granulomatous pleural results without definite evidence of MTB or other granulomatous diseases, but had adjunctive laboratory findings to support TB-P. Other etiologies were established by respective clinical, laboratory, and microbiological results on the basis of granulomatous histological results.
The clinical, blood, radiological, pleural fluid, and histological data were collected and compared between the confirmed and probable TB-P groups and between the confirmed TB-P and non-TB-P groups. All histological slides with pleural granulomas were reviewed by a pulmonary pathologist (TIP) blinded to the final etiologies, and divided into 2 morphological patterns: necrotic and non-necrotic granuloma.13 A standard anti-TB treatment was administered, unless drug modification was necessary because of drug adverse reactions or resistance in patients with TB-P. To evaluate whether anti-TB treatment was appropriate in the probable TB-P group, treatment outcomes were compared between the confirmed and probable TB-P groups. Treatment outcomes were defined on the basis of the revised 2013 World Health Organization definitions of outcomes.5 Treatment success was defined as the sum of cure and treatment completion.
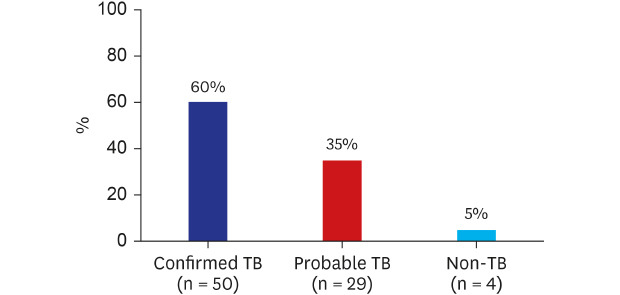
During the study period, pleural biopsy was performed in 146 patients suspected of granulomatous pleural disease. Among these patients, 83 had epithelioid granulomatous inflammation and the remainder had chronic inflammation, necrosis, or fibrosis (n = 46), malignancy (n = 14), and miscellaneous (n = 3). In 83 patients with granulomatous inflammation, pleural biopsy was conducted via closed needle (n = 17), ultrasonography-guided (n = 9), and video-associated thoracoscopic surgery (VATS) (n = 57). The final etiologies of these patients were as follows: confirmed TB-P (n = 50, 60.2%; positive MTB culture [n = 38] and positive TB-PCR [n = 12]), probable TB-P (n = 29, 34.9%), and non-TB-P (n = 4, 4.8%; non-tuberculous mycobacteria [NTM] [n = 2], coccidioidomycosis [n = 1], and rheumatoid arthritis [n = 1]) (Fig. 1). Two NTM pleurisy cases were confirmed by positive culture of M. avium and M. neoaurum in pleural tissue and pleural fluid, respectively. Pleural coccidioidomycosis was confirmed by positive culture of Coccidioides immitis in pleural fluid and tissue. Rheumatoid pleurisy was diagnosed by male, clinical history of rheumatoid arthritis for 20 years, laboratory results (rheumatoid arthritis factor, 295 IU/mL; anti-cyclic citrullinated peptide antibodies, > 500 U/mL), and no evidence of infective pathogens, on the basis of necrotizing granuloma surrounded by palisading histiocytes on VATS pleural biopsy.
Clinical, blood, and radiological findings were not significantly different between the confirmed TB-P and probable TB-P groups (Table 1). Pleural fluid analyses were also not significantly different between the 2 groups, although pleural fluid adenosine deaminase (ADA) levels tended to be higher in the confirmed group than the probable group (90 vs. 73 IU/L; P = 0.065). Almost all patients with probable TB-P had at least one or more findings (positive acid-fast bacillus [AFB] smear, positive interferon-gamma release assay [IGRA], or ADA levels ≥ 50 IU/L with lymphocyte/neutrophil ratio ≥ 0.75) suggesting TB, except for 1 patient who had necrotic granuloma on VATS pleural biopsy. Based on pathological results, the frequency of necrosis within granulomas tended to be greater in the confirmed group than the probable group. However, there was no statistical significance (60% vs. 38%; P = 0.058). In concordance with the diagnostic definition of TB-P classified in this study, the microbiological analyses showed that positive MTB culture and PCR results of pleural fluid and tissue were significantly higher in the confirmed group than in the probable group, as shown in Table 1.
In comparison between the confirmed TB-P and non-TB-P groups, none of the variables were significantly different. This might be attributed mainly to the small number of patients in the non-TB-P group. Nevertheless, it was meaningful that high pleural fluid ADA levels (median, 98 IU/L) were found even in the non-TB-P group. In addition, positive IGRA result (50%) was noted in elderly patients of the non-TB-P group, reflecting the high rate of latent TB infection in the older population of South Korea.6
In comparison between patients with necrotic and non-necrotic granulomas in the confirmed TB-P group, clinical, blood, radiological, and pleural fluid characteristics were not significantly different (data not shown). The underlying immunosuppressive conditions were also not significant between the 2 groups. Not surprisingly, positive culture and TB-PCR results of pleural tissue were higher in patients with necrotic granulomas than non-necrotic granulomas, although there was no significant difference. On the contrary, it was interesting that positive pleural fluid culture tended to be higher in those with non-necrotic granulomas than necrotic granulomas (29% vs. 7%; P = 0.089).
The treatment success rates were 92% (46 of 50) and 97% (28 of 29) in the confirmed and probable TB-P groups, respectively, with no significant difference between the 2 groups (P = 0.307) (Table 1). Two (4%) patients in the confirmed TB-P group died from hepatic failure owing to anti-TB drugs (n = 1) and cancer recurrence (n = 1). Two (4%) patients in the confirmed TB-P group and 1 (3%) patient in the probable TB-P group were lost to follow-up. There was no recurrent case during a similar follow-up duration between the confirmed and probable groups (median, 416 vs. 463 day; P = 0.666). In addition, other diseases were not identified during the treatment and follow-up periods in the probable TB-P group.
The present study shows that almost all cases of granulomatous pleurisy, if probable TB-P cases were included, were attributed to TB in a TB-prevalent country. However, approximately 5% of the cases of granulomatous pleurisy originated from non-TB. In patients with probable TB-P based on granulomatous pathology and clinical correlation, the treatment of standard anti-TB drugs was appropriate in a TB-endemic area and resulted in favorable outcomes. The morphological patterns of pleural granuloma contributed little to the prediction of final diagnosis.
Virtually all granulomatous pleurisies were caused by infection compared with the lung, skin, and lymph node, in which sarcoidosis and infection (TB or fungus) were the most common etiology.123 In addition, the geographic characteristics of a TB-endemic country led to overwhelmingly TB pleurisy. This is likely why many granulomatous lung diseases, such as sarcoidosis, hypersensitivity pneumonitis, and rheumatoid nodules, are rarely involved the pleura.178 Although fungal infection can produce granulomatous pleurisy, it was also rare if not in an endemic area of fungal infection.2 In the current study, 1 patient with coccidioides pleurisy was a foreign immigrant. Thus, our findings suggest that the etiological distribution of granulomatous inflammation may be different between the pleura and other organs.
Although there were few non-TB-P cases in this study, their clinical, laboratory, and pleural fluid characteristics, such as symptom duration, positive IGRA results, and high pleural fluid ADA levels, were similar to those of patients with TB-P. In particular, NTM pleurisy is increasing in both TB-endemic areas as well as worldwide.910 NTM pleurisy can produce positive AFB smear in all specimens, as in TB pleurisy. Definite discrimination between these 2 etiologies can be established only by PCR and culture.1112 Two patients with NTM granulomatous pleurisy were diagnosed by positive NTM culture in pleural tissue or fluid. These findings indicate that clinicians should actively make every effort to find microbiological evidence of potential pathogens in all available specimens, even in cases with granulomatous histological results.
Granulomatous pleural pathology with no evidence of specific granulomatous pleural disease is frequently encountered in clinical practice, as shown by the probable TB-P in this study. Many studies have included this condition as a diagnostic criterion of TB-P.1314 In these cases, correlation with geographic disease prevalence and other laboratory results may lead to a specific diagnosis including infection, similar to this study. Many of our patients with TB-P did not receive full microbiological testing, especially using fresh pleural tissue. The lack of data on these tests was mainly attributed to insufficient tissue samples from closed-needle biopsy used in the early study period and ultrasonography-guided pleural biopsy.
Granulomas are often morphologically categorized as necrotizing and non-necrotizing. Necrotizing granulomas are more likely to be associated with infections.1516 However, infectious pleurisy can show both forms of granulomas, depending on the immune status of the patients. Our study revealed that 40% of confirmed TB-P patients showed non-necrotic granuloma. In addition, almost all (38 of 41) of non-necrotic pleural granulomatous inflammation, when including probable TB-P cases, were attributed to TB. These results were also derived from the rare incidence of non-infectious granulomatous pleural diseases, such as sarcoidosis.78 Thus, the results of this study support that microbiological tests to potential pathogens should be performed for all pleural granuloma, regardless of necrosis, as a diagnostic algorithm shown in Fig. 2.
This study had some limitations including its retrospective, single-center design, and small non-TB-P sample size with inevitable selection bias. In addition, full microbiological tests using pleural tissue were not performed in all patients. Thus, the potential of other etiologies cannot be completely excluded in patients with probable TB-P. Prospective studies with larger sample sizes are warranted.
In conclusion, our study suggests that most pleural granulomatous inflammation was attributable to TB-P in TB-endemic areas and that the granuloma patterns contributed little to the prediction of final diagnosis compared with other organs.
Notes
Author Contributions:
Conceptualization: Kim CH, Lee J.
Data curation: Park S, Park JE, Choi SH, Seo H, Yoo SS, Lee SY, Kim YK, Cha SI, Park JY, Park TI.
Formal analysis: Lee J.
Writing - original draft: Lee J, Kim CH.
Writing - review & editing: Park S, Park JE, Choi SH, Seo H, Yoo SS, Lee SH, Kim YK, Cha SI, Park JY, Park TI.
References
1. Woodard BH, Rosenberg SI, Farnham R, Adams DO. Incidence and nature of primary granulomatous inflammation in surgically removed material. Am J Surg Pathol. 1982; 6(2):119–129. PMID: 7102892.

2. Mukhopadhyay S, Farver CF, Vaszar LT, Dempsey OJ, Popper HH, Mani H, et al. Causes of pulmonary granulomas: a retrospective study of 500 cases from seven countries. J Clin Pathol. 2012; 65(1):51–57. PMID: 22011444.

3. Shah KK, Pritt BS, Alexander MP. Histopathologic review of granulomatous inflammation. J Clin Tuberc Other Mycobact Dis. 2017; 7:1–12. PMID: 31723695.

4. World Health Organization. Global tuberculosis report 2019. Updated 2020. Accessed August 12, 2020. http://www.who.int/tb/data.
5. WHO revised definitions and reporting framework for tuberculosis. Euro Surveill. 2013; 18:20455. PMID: 23611033.
6. Yeon JH, Seong H, Hur H, Park Y, Kim YA, Park YS, et al. Prevalence and risk factors of latent tuberculosis among Korean healthcare workers using whole-blood interferon-gamma release assay. Sci Rep. 2018; 8(1):10113. PMID: 29973678.

7. Judson MA. The clinical features of sarcoidosis: a comprehensive review. Clin Rev Allergy Immunol. 2015; 49(1):63–78. PMID: 25274450.

8. Huggins JT, Doelken P, Sahn SA, King L, Judson MA. Pleural effusions in a series of 181 outpatients with sarcoidosis. Chest. 2006; 129(6):1599–1604. PMID: 16778281.

9. Park S, Jo KW, Lee SD, Kim WS, Shim TS. Clinical characteristics and treatment outcomes of pleural effusions in patients with nontuberculous mycobacterial disease. Respir Med. 2017; 133:36–41. PMID: 29173447.

10. Naito M, Maekura T, Kurahara Y, Tahara M, Ikegami N, Kimura Y, et al. Clinical features of nontuberculous mycobacterial pleurisy: a review of 12 cases. Intern Med. 2018; 57(1):13–16. PMID: 29033435.

11. Ohshimo S, Guzman J, Costabel U, Bonella F. Differential diagnosis of granulomatous lung disease: clues and pitfalls: number 4 in the series "Pathology for the clinician" edited by Peter Dorfmüller and Alberto Cavazza. Eur Respir Rev. 2007; 26(145):170012.
12. Akhter N, Sumalani KK, Chawla D, Ahmed Rizvi N. Comparison between the diagnostic accuracy of Xpert MTB/Rif assay and culture for pleural tuberculosis using tissue biopsy. ERJ Open Res. 2019; 5(3):00065-2019. PMID: 31579677.

13. Che N, Yang X, Liu Z, Li K, Chen X. Rapid detection of cell-free mycobacterium tuberculosis DNA in tuberculous pleural effusion. J Clin Microbiol. 2017; 55(5):1526–1532. PMID: 28275073.

14. Choi H, Chon HR, Kim K, Kim S, Oh KJ, Jeong SH, et al. Clinical and laboratory differences between lymphocyte- and neutrophil-predominant pleural tuberculosis. PLoS One. 2016; 11(10):e0165428. PMID: 27788218.

15. Aubry MC. Necrotizing granulomatous inflammation: what does it mean if your special stains are negative? Mod Pathol. 2012; 25(Suppl 1):S31–8. PMID: 22214968.

16. Karpathiou G, Peoc'h M. Pleura revisited: from histology and pathophysiology to pathology and molecular biology. Clin Respir J. 2019; 13(1):3–13. PMID: 30561890.

Fig. 1
Etiological distribution of pleural granulomatous inflammation in a TB-prevalent country. Non-TB included two non-tuberculous mycobacterial pleurisies, one pleural coccidioidomycosis, and one rheumatoid pleurisy.
TB = tuberculosis.
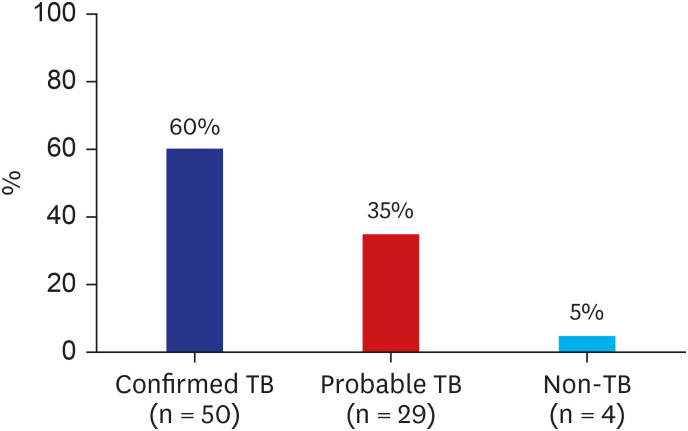
Fig. 2
Diagnostic algorithm of granulomatous pleural diseases in tuberculosis-endemic areas.
PCR = polymerase chain reaction, AFB = acid-fast bacilli, PAS = periodic acid Schiff, GMS = Grocott-Gomori's methenamine silver, MTB = Mycobacterium tuberculosis, NTM = non-tuberculous mycobacteria, IGRA = interferon-gamma release assay, TST = tuberculin skin test, ADA = adenosine deaminase.
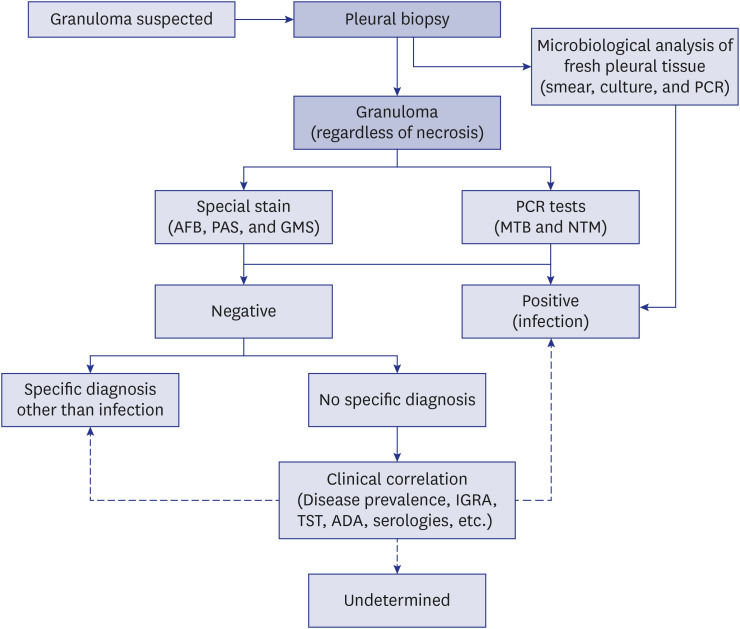
Table 1
Final etiology and characteristics of 83 patients with granulomatous pleurisy
Data are expressed as the median (interquartile range) or number (%). Differences were analyzed by Mann-Whitney test and χ2 or Fisher's exact tests.
ADA = adenosine deaminase, AFB = acid-fast bacilli, CRP = C-reactive protein, GMS = Grocott-Gomori's methenamine silver, IGRA = interferon-gamma release assay, LDH = lactate dehydrogenase, MTB = Mycobacterium tuberculosis, NTM = non-tuberculous mycobacteria, PAS = periodic acid Schiff, PCR = polymerase chain reaction, PF = pleural fluid, PT = pleural tissue, TB = tuberculosis, TLC = total leukocyte cells, WBC = white blood cells.
aComparison between patients with confirmed and probable tuberculosis; bComparison between patients with confirmed tuberculosis and non-tuberculosis; cImmunosuppressive conditions include malignancy, transplantation, end-stage renal disease, advanced liver disease, human immunodeficiency virus infection, and immunosuppressant use.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download