INTRODUCTION
Anorectal malformations (ARMs) are relatively common congenital anomalies in pediatric surgery, with an incidence of 1 in 4,000 to 5,000 live births, and slightly more common in males than in females.
12 Moreover, 50%–60% of patients with ARM are known to have at least one other congenital malformation.
3 Associated anomalies (AAs) were reported to have more AAs in the higher ARM type when Wingspread classification was applied.
4 The most commonly associated organ systems are the genitourinary, spinal, and cardiovascular systems, and many studies have evaluated AAs in patients with ARM.
45678 However, some studies are limited by their small sample sizes.
67 Other studies used a classification of ARM that most current physicians are not familiar with.
48 In addition, in some studies, the protocolized screening examination for AA was not performed in all the patients.
8
Associated congenital anomalies can lead to overall mortality and contribute to serious morbidity. Among these congenital anomalies, the major ones are generally defined as structural abnormalities of surgical, medical, functional, or cosmetic importance. Many studies have assessed AAs in patients with ARM, but to our knowledge, no studies have yet classified AAs as major and minor congenital anomalies.
This study analyzed the incidence of congenital anomalies associated with the subtypes of ARM classified in accordance with the Krickenbeck classification in a relatively large number of patients enrolled at a single center and the differences in the incidence rates of major and minor AAs according to organ system among patients with ARM.
RESULTS
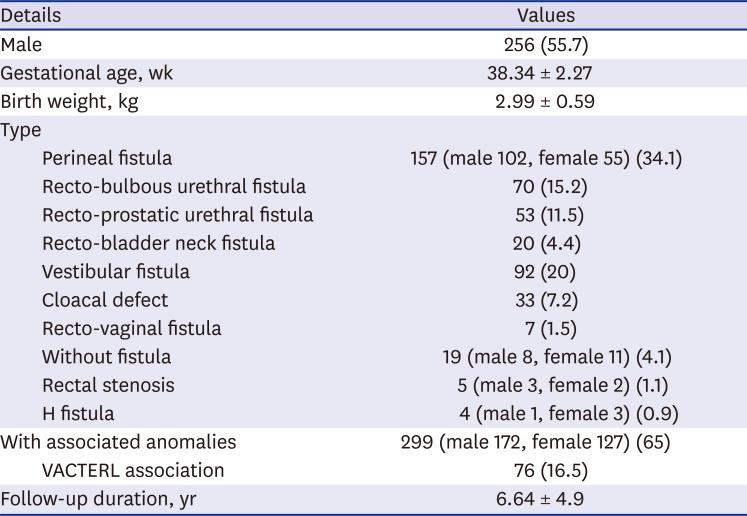
Of the 460 patients, 256 (55.7%) were male, with a mean gestational age of 38.34 ± 2.27 weeks and a mean birth weight of 2.99 ± 0.59 kg. The most common ARM subtype was perineal fistula (157, 34.1%), which was found in 102 male patients. The second most common subtype was the vestibular fistula, which was found in 92 patients (20%). Of the total patients, 299 (65%) had at least one AA. No statistical significance was found in the presence of AAs between boys and girls, although AAs were slightly more likely to occur in boys than in girls (67.2% in boys and 62.2% in girls,
P = 0.281) (
Table 1).
Table 1
Characteristics of the patients
|
Details |
Values |
|
Male |
256 (55.7) |
|
Gestational age, wk |
38.34 ± 2.27 |
|
Birth weight, kg |
2.99 ± 0.59 |
|
Type |
|
|
Perineal fistula |
157 (male 102, female 55) (34.1) |
|
Recto-bulbous urethral fistula |
70 (15.2) |
|
Recto-prostatic urethral fistula |
53 (11.5) |
|
Recto-bladder neck fistula |
20 (4.4) |
|
Vestibular fistula |
92 (20) |
|
Cloacal defect |
33 (7.2) |
|
Recto-vaginal fistula |
7 (1.5) |
|
Without fistula |
19 (male 8, female 11) (4.1) |
|
Rectal stenosis |
5 (male 3, female 2) (1.1) |
|
H fistula |
4 (male 1, female 3) (0.9) |
|
With associated anomalies |
299 (male 172, female 127) (65) |
|
VACTERL association |
76 (16.5) |
|
Follow-up duration, yr |
6.64 ± 4.9 |

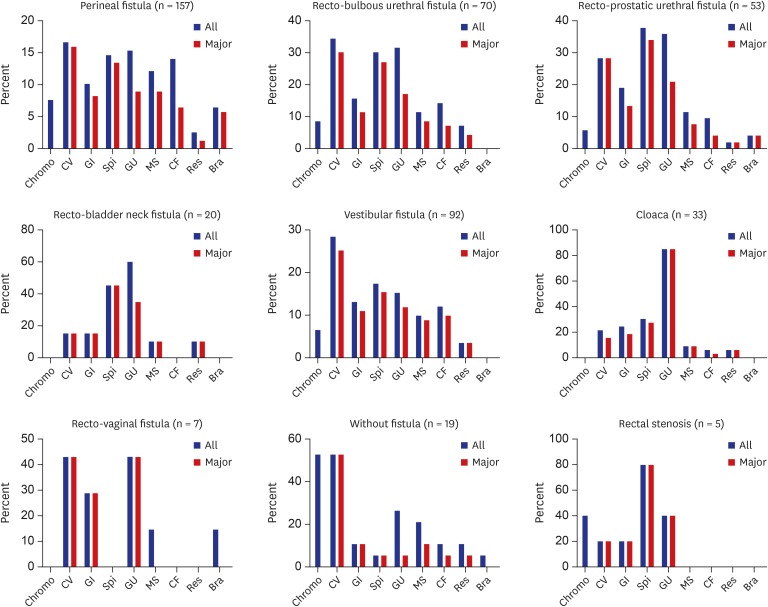
According to organ system, AAs were most common in genitourinary anomalies (28%), followed by cardiovascular (25%) and spinal/vertebral anomalies (22.6%). However, major AAs were most common in cardiovascular anomalies (23%), followed by spinal/vertebral (20.6%) and genitourinary anomalies (19.3%) (
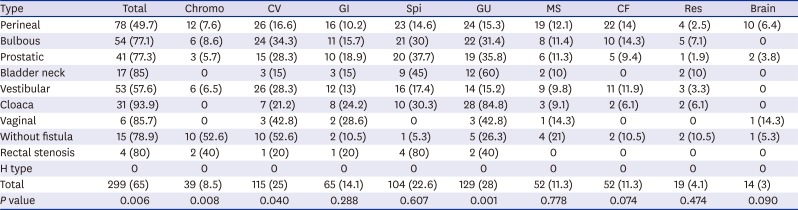
Fig. 1). The ARM subtype analysis revealed that 93.9% of cloaca had an AA, followed by rectovaginal (85.7%) and recto-bladder neck fistula (85%). In the perineal fistula, 49.7% of the patients had AAs and 57.6% of vestibular fistula had AAs. In H type, none of the patients had AAs. Some statistically significant difference was observed in the incidence of AAs among the subtypes. Chromosomal (
P = 0.008) and cardiovascular anomalies (
P = 0.04) showed the highest incidence in the group without fistulas, while genitourinary anomaly (
P = 0.001) was the most frequent in the group with cloaca (
Table 2).
Fig. 1
Incidence of associated anomalies in anorectal malformation according to organ system.
Chromo = chromosomal, CV = cardiovascular, GI = gastrointestinal, Spi = spinal/vertebral, GU = genitourinary, MS = musculoskeletal, CF = craniofacial, Res = respiratory, Bra = brain.
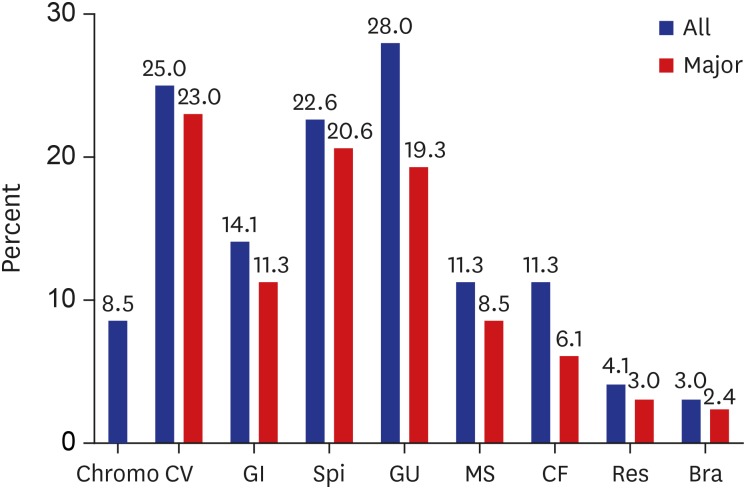

Table 2
Incidence of anomalies associated with the anorectal malformation subtypes
|
Type |
Total |
Chromo |
CV |
GI |
Spi |
GU |
MS |
CF |
Res |
Brain |
|
Perineal |
78 (49.7) |
12 (7.6) |
26 (16.6) |
16 (10.2) |
23 (14.6) |
24 (15.3) |
19 (12.1) |
22 (14) |
4 (2.5) |
10 (6.4) |
|
Bulbous |
54 (77.1) |
6 (8.6) |
24 (34.3) |
11 (15.7) |
21 (30) |
22 (31.4) |
8 (11.4) |
10 (14.3) |
5 (7.1) |
0 |
|
Prostatic |
41 (77.3) |
3 (5.7) |
15 (28.3) |
10 (18.9) |
20 (37.7) |
19 (35.8) |
6 (11.3) |
5 (9.4) |
1 (1.9) |
2 (3.8) |
|
Bladder neck |
17 (85) |
0 |
3 (15) |
3 (15) |
9 (45) |
12 (60) |
2 (10) |
0 |
2 (10) |
0 |
|
Vestibular |
53 (57.6) |
6 (6.5) |
26 (28.3) |
12 (13) |
16 (17.4) |
14 (15.2) |
9 (9.8) |
11 (11.9) |
3 (3.3) |
0 |
|
Cloaca |
31 (93.9) |
0 |
7 (21.2) |
8 (24.2) |
10 (30.3) |
28 (84.8) |
3 (9.1) |
2 (6.1) |
2 (6.1) |
0 |
|
Vaginal |
6 (85.7) |
0 |
3 (42.8) |
2 (28.6) |
0 |
3 (42.8) |
1 (14.3) |
0 |
0 |
1 (14.3) |
|
Without fistula |
15 (78.9) |
10 (52.6) |
10 (52.6) |
2 (10.5) |
1 (5.3) |
5 (26.3) |
4 (21) |
2 (10.5) |
2 (10.5) |
1 (5.3) |
|
Rectal stenosis |
4 (80) |
2 (40) |
1 (20) |
1 (20) |
4 (80) |
2 (40) |
0 |
0 |
0 |
0 |
|
H type |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Total |
299 (65) |
39 (8.5) |
115 (25) |
65 (14.1) |
104 (22.6) |
129 (28) |
52 (11.3) |
52 (11.3) |
19 (4.1) |
14 (3) |
|
P value |
0.006 |
0.008 |
0.040 |
0.288 |
0.607 |
0.001 |
0.778 |
0.074 |
0.474 |
0.090 |

In the analysis of major AAs, the difference in incidence between the subtypes was similar to the total AA incidence. Cloaca (93.9%) had the highest incidence, followed by rectovaginal fistula (85.7%) and rectal stenosis (80%). Major cardiovascular anomaly showed the highest incidence in the without fistulas, but the difference between subtypes was not statistically significant (
P = 0.062). However, the incidence of the subtypes in the major genitourinary anomalies was statistically significant (
P < 0.001) (
Table 3).
Table 3
Incidence of major anomalies associated with the anorectal malformation subtypes
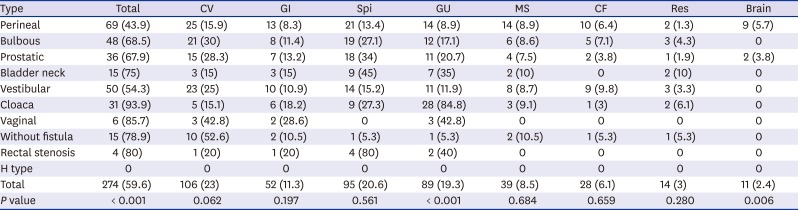
|
Type |
Total |
CV |
GI |
Spi |
GU |
MS |
CF |
Res |
Brain |
|
Perineal |
69 (43.9) |
25 (15.9) |
13 (8.3) |
21 (13.4) |
14 (8.9) |
14 (8.9) |
10 (6.4) |
2 (1.3) |
9 (5.7) |
|
Bulbous |
48 (68.5) |
21 (30) |
8 (11.4) |
19 (27.1) |
12 (17.1) |
6 (8.6) |
5 (7.1) |
3 (4.3) |
0 |
|
Prostatic |
36 (67.9) |
15 (28.3) |
7 (13.2) |
18 (34) |
11 (20.7) |
4 (7.5) |
2 (3.8) |
1 (1.9) |
2 (3.8) |
|
Bladder neck |
15 (75) |
3 (15) |
3 (15) |
9 (45) |
7 (35) |
2 (10) |
0 |
2 (10) |
0 |
|
Vestibular |
50 (54.3) |
23 (25) |
10 (10.9) |
14 (15.2) |
11 (11.9) |
8 (8.7) |
9 (9.8) |
3 (3.3) |
0 |
|
Cloaca |
31 (93.9) |
5 (15.1) |
6 (18.2) |
9 (27.3) |
28 (84.8) |
3 (9.1) |
1 (3) |
2 (6.1) |
0 |
|
Vaginal |
6 (85.7) |
3 (42.8) |
2 (28.6) |
0 |
3 (42.8) |
0 |
0 |
0 |
0 |
|
Without fistula |
15 (78.9) |
10 (52.6) |
2 (10.5) |
1 (5.3) |
1 (5.3) |
2 (10.5) |
1 (5.3) |
1 (5.3) |
0 |
|
Rectal stenosis |
4 (80) |
1 (20) |
1 (20) |
4 (80) |
2 (40) |
0 |
0 |
0 |
0 |
|
H type |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Total |
274 (59.6) |
106 (23) |
52 (11.3) |
95 (20.6) |
89 (19.3) |
39 (8.5) |
28 (6.1) |
14 (3) |
11 (2.4) |
|
P value |
< 0.001 |
0.062 |
0.197 |
0.561 |
< 0.001 |
0.684 |
0.659 |
0.280 |
0.006 |

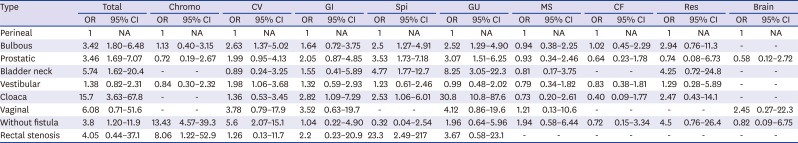
In the comparison of the ORs between the ARM subtypes using the cases of perineal fistula as the base group, cloaca (OR, 15.7) and recto-bladder neck fistula (OR, 5.74) showed the highest OR in the total AAs. Chromosomal anomaly (OR, 13.43) and cardiovascular anomaly (OR, 5.6) showed the highest OR in the without fistula. The category of gastrointestinal anomaly showed a significant OR only in cloaca (OR, 2.82). Spinal/vertebral anomaly showed the highest OR in rectal stenosis (OR, 23.3), followed by recto-bladder neck fistula (OR, 4.77) and recto-prostatic urethral fistula (OR, 3.53). Genitourinary anomaly showed the highest OR in cloaca (OR, 30.8), followed by recto-bladder neck fistula (OR, 8.25) (
Table 4).
Table 4
Comparison of associated anomalies between anorectal malformation subtypes with perineal fistula cases as the base group
|
Type |
Total |
Chromo |
CV |
GI |
Spi |
GU |
MS |
CF |
Res |
Brain |
|
OR |
95% CI |
OR |
95% CI |
OR |
95% CI |
OR |
95% CI |
OR |
95% CI |
OR |
95% CI |
OR |
95% CI |
OR |
95% CI |
OR |
95% CI |
OR |
95% CI |
|
Perineal |
1 |
NA |
1 |
NA |
1 |
NA |
1 |
NA |
1 |
NA |
1 |
NA |
1 |
NA |
1 |
NA |
1 |
NA |
1 |
NA |
|
Bulbous |
3.42 |
1.80–6.48 |
1.13 |
0.40–3.15 |
2.63 |
1.37–5.02 |
1.64 |
0.72–3.75 |
2.5 |
1.27–4.91 |
2.52 |
1.29–4.90 |
0.94 |
0.38–2.25 |
1.02 |
0.45–2.29 |
2.94 |
0.76–11.3 |
- |
- |
|
Prostatic |
3.46 |
1.69–7.07 |
0.72 |
0.19–2.67 |
1.99 |
0.95–4.13 |
2.05 |
0.87–4.85 |
3.53 |
1.73–7.18 |
3.07 |
1.51–6.25 |
0.93 |
0.34–2.46 |
0.64 |
0.23–1.78 |
0.74 |
0.08–6.73 |
0.58 |
0.12–2.72 |
|
Bladder neck |
5.74 |
1.62–20.4 |
- |
- |
0.89 |
0.24–3.25 |
1.55 |
0.41–5.89 |
4.77 |
1.77–12.7 |
8.25 |
3.05–22.3 |
0.81 |
0.17–3.75 |
- |
- |
4.25 |
0.72–24.8 |
- |
- |
|
Vestibular |
1.38 |
0.82–2.31 |
0.84 |
0.30–2.32 |
1.98 |
1.06–3.68 |
1.32 |
0.59–2.93 |
1.23 |
0.61–2.46 |
0.99 |
0.48–2.02 |
0.79 |
0.34–1.82 |
0.83 |
0.38–1.81 |
1.29 |
0.28–5.89 |
- |
- |
|
Cloaca |
15.7 |
3.63–67.8 |
- |
- |
1.36 |
0.53–3.45 |
2.82 |
1.09–7.29 |
2.53 |
1.06–6.01 |
30.8 |
10.8–87.6 |
0.73 |
0.20–2.61 |
0.40 |
0.09–1.77 |
2.47 |
0.43–14.1 |
- |
- |
|
Vaginal |
6.08 |
0.71–51.6 |
- |
- |
3.78 |
0.79–17.9 |
3.52 |
0.63–19.7 |
- |
- |
4.12 |
0.86–19.6 |
1.21 |
0.13–10.6 |
- |
- |
- |
- |
2.45 |
0.27–22.3 |
|
Without fistula |
3.8 |
1.20–11.9 |
13.43 |
4.57–39.3 |
5.6 |
2.07–15.1 |
1.04 |
0.22–4.90 |
0.32 |
0.04–2.54 |
1.96 |
0.64–5.96 |
1.94 |
0.58–6.44 |
0.72 |
0.15–3.34 |
4.5 |
0.76–26.4 |
0.82 |
0.09–6.75 |
|
Rectal stenosis |
4.05 |
0.44–37.1 |
8.06 |
1.22–52.9 |
1.26 |
0.13–11.7 |
2.2 |
0.23–20.9 |
23.3 |
2.49–217 |
3.67 |
0.58–23.1 |
- |
- |
- |
- |
- |
- |
- |
- |

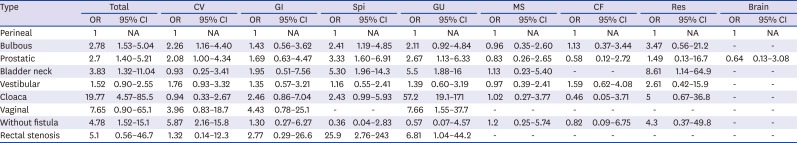
In the analysis of total major AAs, cloaca (OR, 19.77) showed the highest OR, followed by no fistula (OR, 4.78) and recto-bladder neck fistula (OR, 3.83). Cardiovascular major AA showed the highest OR in the without fistula (OR, 5.87), and gastrointestinal major AA showed no statistical significance for each subtype. Spinal/vertebral and genitourinary anomalies showed the highest OR in rectal stenosis and cloaca, respectively (
Table 5).
Table 5
Comparison of major associated anomalies between the anorectal malformation subtypes with perineal fistula cases as the base group
|
Type |
Total |
CV |
GI |
Spi |
GU |
MS |
CF |
Res |
Brain |
|
OR |
95% CI |
OR |
95% CI |
OR |
95% CI |
OR |
95% CI |
OR |
95% CI |
OR |
95% CI |
OR |
95% CI |
OR |
95% CI |
OR |
95% CI |
|
Perineal |
1 |
NA |
1 |
NA |
1 |
NA |
1 |
NA |
1 |
NA |
1 |
NA |
1 |
NA |
1 |
NA |
1 |
NA |
|
Bulbous |
2.78 |
1.53–5.04 |
2.26 |
1.16–4.40 |
1.43 |
0.56–3.62 |
2.41 |
1.19–4.85 |
2.11 |
0.92–4.84 |
0.96 |
0.35–2.60 |
1.13 |
0.37–3.44 |
3.47 |
0.56–21.2 |
- |
- |
|
Prostatic |
2.7 |
1.40–5.21 |
2.08 |
1.00–4.34 |
1.69 |
0.63–4.47 |
3.33 |
1.60–6.91 |
2.67 |
1.13–6.33 |
0.83 |
0.26–2.65 |
0.58 |
0.12–2.72 |
1.49 |
0.13–16.7 |
0.64 |
0.13–3.08 |
|
Bladder neck |
3.83 |
1.32–11.04 |
0.93 |
0.25–3.41 |
1.95 |
0.51–7.56 |
5.30 |
1.96–14.3 |
5.5 |
1.88–16 |
1.13 |
0.23–5.40 |
- |
- |
8.61 |
1.14–64.9 |
- |
- |
|
Vestibular |
1.52 |
0.90–2.55 |
1.76 |
0.93–3.32 |
1.35 |
0.57–3.21 |
1.16 |
0.55–2.41 |
1.39 |
0.60–3.19 |
0.97 |
0.39–2.41 |
1.59 |
0.62–4.08 |
2.61 |
0.42–15.9 |
- |
- |
|
Cloaca |
19.77 |
4.57–85.5 |
0.94 |
0.33–2.67 |
2.46 |
0.86–7.04 |
2.43 |
0.99–5.93 |
57.2 |
19.1–171 |
1.02 |
0.27–3.77 |
0.46 |
0.05–3.71 |
5 |
0.67–36.8 |
- |
- |
|
Vaginal |
7.65 |
0.90–65.1 |
3.96 |
0.83–18.7 |
4.43 |
0.78–25.1 |
- |
- |
7.66 |
1.55–37.7 |
- |
- |
- |
- |
- |
- |
- |
- |
|
Without fistula |
4.78 |
1.52–15.1 |
5.87 |
2.16–15.8 |
1.30 |
0.27–6.27 |
0.36 |
0.04–2.83 |
0.57 |
0.07–4.57 |
1.2 |
0.25–5.74 |
0.82 |
0.09–6.75 |
4.3 |
0.37–49.8 |
- |
- |
|
Rectal stenosis |
5.1 |
0.56–46.7 |
1.32 |
0.14–12.3 |
2.77 |
0.29–26.6 |
25.9 |
2.76–243 |
6.81 |
1.04–44.2 |
- |
- |
- |
- |
- |
- |
- |
- |

DISCUSSION
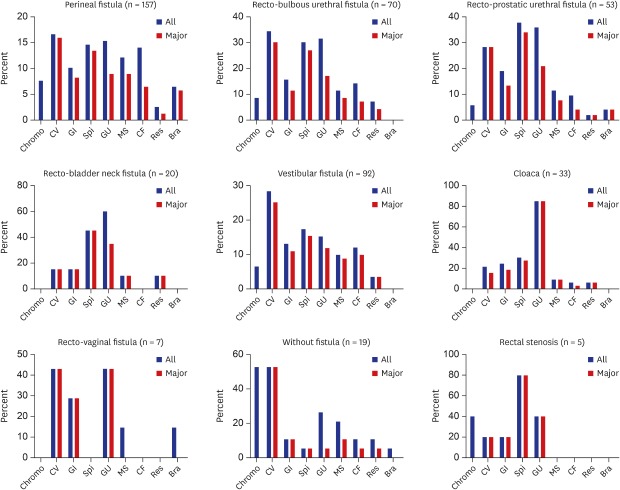
Our study was conducted with a relatively large number of patients with ARM in a single center and facilitated communication with other physicians and researchers by applying the Krickenbeck classification, which is currently the most frequently used ARM classification system (
Fig. 2). Moreover, our protocolized screening examination for AAs has been applied since 1999, which is a strength of this study. However, the spinal screening examination in our center has been applied strictly to all patients with ARM since January 2002. In our institution, newborns with sacral dimple or signs of occult or apert spinal dysraphism, such as incontinence, loss of sensation, and extremity weakness or paralysis, were examined with spinal ultrasonography or MRI because spinal anomaly might be present. In our study, 89 patients underwent anorectoplasty between 1999 and 2001, and 51 of them underwent spinal examination, but 38 (8.3% in our study population) did not. Spinal cord anomaly was found in 9 of the 51 patients. The ARM subtypes of the 38 patients who had not undergone spinal screening examination were perineal fistula in 21, recto-urethral fistula in 6, no fistula in 3, vestibular fistula in 6, recto-vaginal fistula in 1, and H-type in 1. However, these did not belong to either the cloaca or recto-bladder neck fistula subtype. Therefore, excluding these 38 patients would have affected the actual incidence and proportion of ARM subtypes in this study. It would also have affected the actual incidence of AAs other than spinal anomaly. Therefore, we conducted a study including these 38 patients. The incidence of spinal anomaly in our study may be underestimated owing to these 38 patients.
Fig. 2
Incidence of associated anomalies according to anorectal malformation subtype.
Chromo = chromosomal, CV = cardiovascular, GI = gastrointestinal, Spi = spinal/vertebra, GU = genitourinary, MS = musculoskeletal, CF = craniofacial, Res = respiratory, Bra = brain.

In general, major congenital anomalies define structural anomalies such as significant medical, social, or cosmetic consequences for the affected individual, and usually require medical or surgical intervention. Conversely, minor congenital anomalies are structural changes that pose no significant health problem in the neonatal period and tend to have limited social or cosmetic consequences for the affected individual.
11 A large number of congenital anomalies are difficult to classify by only two stages, major and minor. Congenital anomalies such as anencephaly and gastroschisis, which are lethal or require surgical correction, may be easily classified as major congenital anomalies. However, congenital malformations such as atrial septal defect or intestinal malrotation are sometimes fatal but may not cause any life-threatening symptoms. Therefore, major and minor congenital anomalies are difficult to distinguish. Here, minor congenital anomalies of congenital malformations were identified using the EUROCAT Guide 1.4 definition,
1 and major AAs were defined as all malformations other than minor congenital anomalies in this study. The incidence of major AA according to organ system did not differ significantly from the total AA incidence, including minor cases, perhaps because a small number of congenital malformations were classified as minor anomalies. Indexes dividing major and minor congenital anomalies for each organ system more clearly would have made our study more meaningful. We described the major AAs in patients with ARM by applying the concept of major and minor congenital malformations. Our data will be of great significance in reporting major congenital anomalies that should be considered more carefully in the AAs of ARM.
The time when the screening examination of AAs in patients with ARM was first systematically performed is unclear. To understand ARM-related congenital anomalies, the VATER/VACTERL association must be understood. The VATER association, which was first described in 1973, is a spectrum of AAs that refers to patients with coexistence of at least three of the anomalies of the vertebrae, ARMs, esophageal atresia/tracheoesophageal fistula, and radial and renal anomalies.
12 Shortly thereafter, cardiac and limb malformations were added to the anomalous feature of the VATER association, which was called the VACTERL association.
13 Therefore, patients with suspected VACTERL association should undergo diagnostic tests (radiography, echocardiography, and abdominal ultrasonography including the kidney, etc.) based on careful physical examination. The incidence of VACTERL association in patients with ARM is known to be 15.4%–17.8%.
1415 Thus, for decades, patients with ARM would have been given diagnostic tests for the VACTERL association. In our study, 76 patients (16.5%) were eligible for VACTERL association.
Here, patients with spinal cord or vertebral anomalies, including sacral anomalies, accounted for 22.6% of all patients with ARM. The tethered cord refers to the state in which the conus medullaris is located below the L2 vertebra and is associated with the anomalies of spinal dysraphism. Levitt et al.
16 reported that 24% of patients with ARM had a tethered cord. In this study, 69 patients (15%) presented with a tethered cord when evaluated on the basis of the above-mentioned diagnostic criteria of tethered cord. The prevalence of tethered cord, in fact, reported differed depending on the definition of tethered cord and the diagnostic tool used. In a recent report of pediatric surgeons in 24 European centers (members of the ARM-Net Consortium), the overall prevalence of tethered cord in patients with ARM was 46% in the respondents who reported a prevalence of < 15%, 29% in respondents who reported 15% to 30%, and 4% in the respondents who reported 30% to 45%.
17 Several reports that diagnosed spinal anomaly by performing MRI on all patients with ARM reported that the prevalence of tethered cord or spinal anomaly ranged from 35% to 60%.
18192021 Other reports indicated that the prevalence of spinal anomaly varies according to ARM subtype. In a study of 416 patients with ARM in 2004, the incidence of spinal cord anomaly was found to be 4.4% in the low type and 10% in the high type, showing a significant difference (
P = 0.04).
8 Other studies also reported that the prevalence of spinal anomalies was higher in the high ARM type than in the low ARM type (31.2% vs. 50%
18 and 11.4% vs. 47.3%
21). On the other hand, in some studies, the prevalence of tethered spinal cord was higher in the low ARM type than in the high ARM type (50% vs. 26.8%
19 and 53.3% vs. 38.5%
20). In our study, tethered cord was found in 10.8% of the low ARM type cases and 17.3% of the high ARM type cases, without statistical significance (
P = 0.077). We therefore believe that screen examination for spinal/vertebral anomaly should be performed on all ARM patients.
The association between the genitourinary system and ARM has long been known.
22 In 1971, a survey by the members of the surgical section of the American Academy of Pediatrics reported that 26% of 1,166 patients with ARM had urological malformations.
23 Renal agenesis, renal dysplasia, and vesicoureteral reflux (VUR) are the most common anomalies that can affect the morbidity or mortality of patients with genitourinary anomaly.
24 Abdominal ultrasonography can easily detect renal abnormalities, but VUR cannot be easily diagnosed without VCUG. Therefore, it is argued that VCUG should be applied to all patients with ARM.
2526 Previous studies showed that the incidence of VUR in patients with ARM was varied, ranging from 2.3% to 47%.
52427282930 One recent study reported that VCUR was performed in 133 patients with ARM, of whom 41 (31%) had VUR.
31 Of the 41 patients with VUR in this study, 56% had a VUR of grade ≥ 3. This study also showed that VUR is not related to the location of the fistula. In our study, VUR was confirmed in 46 patients (10%) and 30 patients (65.2%) with grade ≥ 3 VUR. The incidence of VUR according to ARM subtype was 3.2% in perineal fistula (5), 11.4% in recto-bulbous urethral fistula (8), 17% in recto-prostatic urethral fistula (9), 20% in recto-bladder neck fistula (4), 5.4% in vestibular fistula (5), 30.3% in cloaca (10), 15.8% in no fistula (3), and 40% in rectal stenosis (2). In our study, fistula location and VUR incidence were statistically significant when cross-tabulation analysis was performed (
P = 0.006). However, in our study, the VUR incidence in perineal and vestibular fistulas may be underestimated because VCUG was performed only in some patients with perineal and vestibular fistulas. In many cases, reflux nephropathy can be prevented by medical therapy, and low-grade VUR tends to improve over time.
2629 Although the incidence of VUR is not high among patients with ARM, considering that a considerable number of VURs were grade ≥ 3, we believe that VCUG should be performed as an initial screening test in all patients with ARM.
Many studies have evaluated musculoskeletal, craniofacial, respiratory, or brain anomalies in patients with ARM who have AAs. However, no consensus has been reached on the screening test for these organ systems, such as limb radiography or brain imaging, probably because of the relatively low incidence as compared with those of heart, spinal, genitourinary, and gastrointestinal anomalies, and anomaly of these organ systems do not significantly affect the morbidity or corrective procedure of ARM. Recently, a study of 506 patients with ARM reported that only 15.2% of patients underwent limb radiography or ultrasonography as a screening test for limb anomalies.
32 In our study, radiography or ultrasonography was used for screening limb anomalies in only 16.3% of the patients, most of whom were patients with limb anomalies that could be identified on visual examination.
The retrospective design is one limitation of the present study. Also, the protocol for evaluation of AAs is not consistent over study period. These are the weak points of our study. If a prospective study complements these points, it would be a more powerful study. As mentioned in the methods, to analyze the difference in incidence of AAs, we calculated ORs using cases of perineal fistula as the base group. The reason why we used the perineal fistula patients as the base group was that there were lot of patients and incidence of AAs was the lowest in this group. If there was an analysis of congenital anomaly in healthy children born during the study period, and if it used as a base group, it would be a better study.
In conclusion, of the patients, 65% had at least one anomaly and 59.6% had major AAs. The most common AAs were genitourinary, cardiovascular, and spinal/vertebral anomalies, and the major AAs were cardiovascular, spinal/vertebral, and genitourinary anomalies, in that order. The subtype with the highest AA incidence was cloaca (93.9%). By organ system, cardiovascular anomaly was most frequent in the without fistula, and gastrointestinal anomaly was most frequent in recto-vaginal fistula. Moreover, spinal/vertebral anomaly was more frequent in rectal stenosis and recto-bladder neck, and genitourinary anomaly was most frequent in cloaca. These data will be effective for predicting AAs in patients with ARM. Altogether, all patients with ARM should undergo a thorough systematic evaluation.










 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download