1. Koide M, Hamada J, Hagiwara Y, Kanazawa K, Suzuki K. A thickened coracohumeral ligament and superomedial capsule limit internal rotation of the shoulder joint: report of three cases. Case Rep Orthop. 2016; 2016:9384974. PMID:
27123353.

2. Li JQ, Tang KL, Wang J, Li QY, Xu HT, Yang HF, et al. MRI findings for frozen shoulder evaluation: is the thickness of the coracohumeral ligament a valuable diagnostic tool? PLoS One. 2011; 6(12):e28704. PMID:
22163326.

3. Homsi C, Bordalo-Rodrigues M, da Silva JJ, Stump XM. Ultrasound in adhesive capsulitis of the shoulder: Is assessment of the coracohumeral ligament a valuable diagnostic tool? Skeletal Radiol. 2006; 35(9):673–678. PMID:
16724200.

4. Neer CS 2nd, Satterlee CC, Dalsey RM, Flatow EL. The anatomy and potential effects of contracture of the coracohumeral ligament. Clin Orthop Relat Res. 1992; (280):182–185.

5. Ferrari DA. Capsular ligaments of the shoulder. Anatomical and functional study of the anterior superior capsule. Am J Sports Med. 1990; 18(1):20–24. PMID:
2301686.
6. Gondim Teixeira PA, Balaj C, Chanson A, Lecocq S, Louis M, Blum A. Adhesive capsulitis of the shoulder: value of inferior glenohumeral ligament signal changes on T2-weighted fat-saturated images. AJR Am J Roentgenol. 2012; 198(6):W589–W596. PMID:
22623575.
7. Lee KH, Park HJ, Lee SY, Youn IY, Kim E, Park JH, et al. Adhesive capsulitis of the shoulder joint: value of glenohumeral distance on magnetic resonance arthrography. J Comput Assist Tomogr. 2017; 41(1):116–120. PMID:
27560018.
8. Loveday DT, Johnston P, Arthur A, Tytherleigh-Strong GM. Frozen shoulder: a clinical review. Br J Hosp Med (Lond). 2009; 70(5):276–278. PMID:
19451871.

9. Uppal HS, Evans JP, Smith C. Frozen shoulder: a systematic review of therapeutic options. World J Orthop. 2015; 6(2):263–268. PMID:
25793166.

10. Wong PL, Tan HC. A review on frozen shoulder. Singapore Med J. 2010; 51(9):694–697. PMID:
20938608.
11. Neviaser AS, Hannafin JA. Adhesive capsulitis: a review of current treatment. Am J Sports Med. 2010; 38(11):2346–2356. PMID:
20110457.
12. Arai R, Nimura A, Yamaguchi K, Yoshimura H, Sugaya H, Saji T, et al. The anatomy of the coracohumeral ligament and its relation to the subscapularis muscle. J Shoulder Elbow Surg. 2014; 23(10):1575–1581. PMID:
24766789.

13. Clark JM, Harryman DT 2nd. Tendons, ligaments, and capsule of the rotator cuff. Gross and microscopic anatomy. J Bone Joint Surg Am. 1992; 74(5):713–725. PMID:
1624486.

14. Harryman DT 2nd, Sidles JA, Harris SL, Matsen FA 3rd. The role of the rotator interval capsule in passive motion and stability of the shoulder. J Bone Joint Surg Am. 1992; 74(1):53–66. PMID:
1734014.

15. Emig EW, Schweitzer ME, Karasick D, Lubowitz J. Adhesive capsulitis of the shoulder: MR diagnosis. AJR Am J Roentgenol. 1995; 164(6):1457–1459. PMID:
7754892.

16. Itoi E, Arce G, Bain GI, Diercks RL, Guttmann D, Imhoff AB, et al. Shoulder stiffness: current concepts and concerns. Arthroscopy. 2016; 32(7):1402–1414. PMID:
27180923.

17. Chellathurai A, Subbiah K, Elangovan A, Kannappan S. Adhesive capsulitis: MRI correlation with clinical stages and proposal of MRI staging. Indian J Radiol Imaging. 2019; 29(1):19–24. PMID:
31000937.

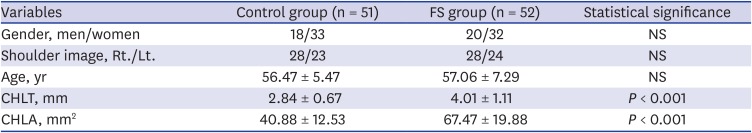
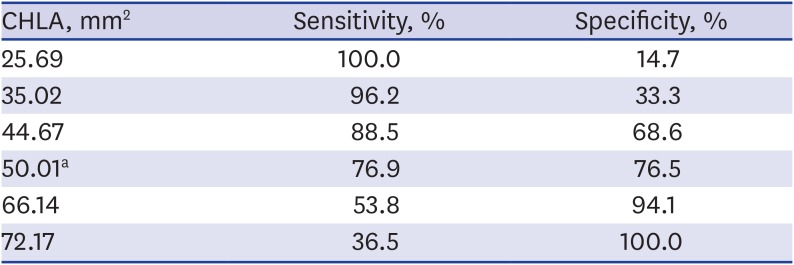
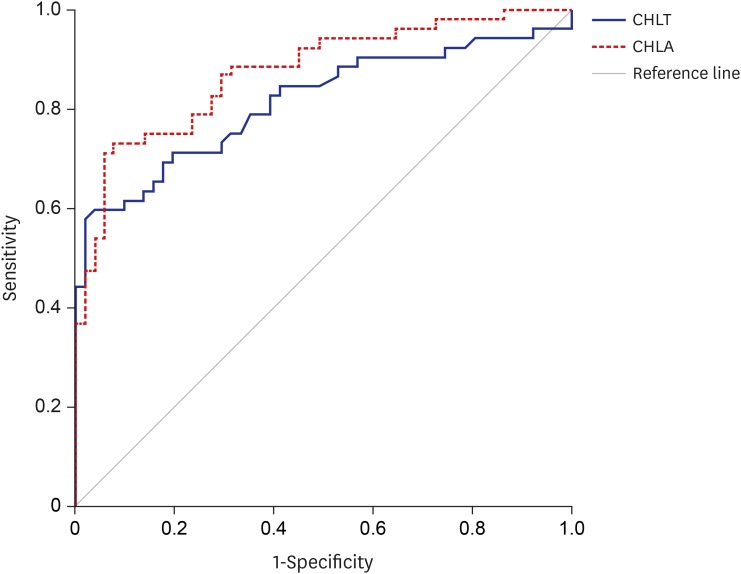
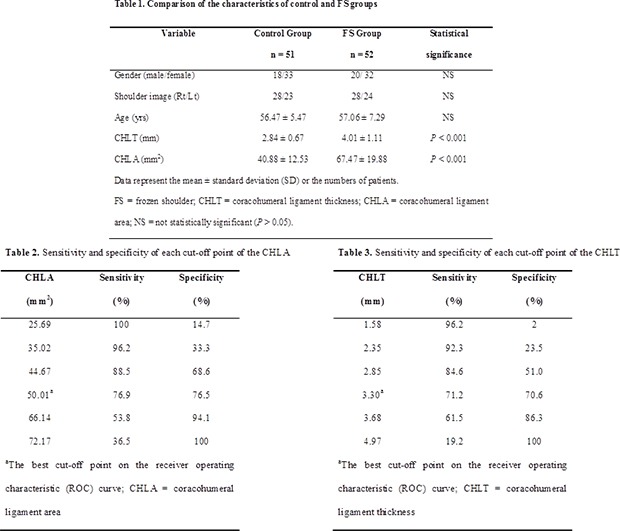
18. Park SH, Goo JM, Jo CH. Receiver operating characteristic (ROC) curve: practical review for radiologists. Korean J Radiol. 2004; 5(1):11–18. PMID:
15064554.

19. Dias R, Cutts S, Massoud S. Frozen shoulder. BMJ. 2005; 331(7530):1453–1456. PMID:
16356983.

20. Lefevre-Colau MM, Drapé JL, Fayad F, Rannou F, Diche T, Minvielle F, et al. Magnetic resonance imaging of shoulders with idiopathic adhesive capsulitis: reliability of measures. Eur Radiol. 2005; 15(12):2415–2422. PMID:
16003508.

21. Jung JY, Jee WH, Chun HJ, Kim YS, Chung YG, Kim JM. Adhesive capsulitis of the shoulder: evaluation with MR arthrography. Eur Radiol. 2006; 16(4):791–796. PMID:
16228212.

22. Sofka CM, Ciavarra GA, Hannafin JA, Cordasco FA, Potter HG. Magnetic resonance imaging of adhesive capsulitis: correlation with clinical staging. HSS J. 2008; 4(2):164–169. PMID:
18815860.

23. Hand GC, Athanasou NA, Matthews T, Carr AJ. The pathology of frozen shoulder. J Bone Joint Surg Br. 2007; 89(7):928–932. PMID:
17673588.

24. Omari A, Bunker TD. Open surgical release for frozen shoulder: surgical findings and results of the release. J Shoulder Elbow Surg. 2001; 10(4):353–357. PMID:
11517365.

25. Kanazawa K, Hagiwara Y, Kawai N, Sekiguchi T, Koide M, Ando A, et al. Correlations of coracohumeral ligament and range of motion restriction in patients with recurrent anterior glenohumeral instability evaluated by magnetic resonance arthrography. J Shoulder Elbow Surg. 2017; 26(2):233–240. PMID:
27814944.

26. Hagiwara Y, Sekiguchi T, Ando A, Kanazawa K, Koide M, Hamada J, et al. Effects of arthroscopic coracohumeral ligament release on range of motion for patients with frozen shoulder. Open Orthop J. 2018; 12(1):373–379. PMID:
30288192.

27. Yukata K, Goto T, Sakai T, Fujii H, Hamawaki J, Yasui N. Ultrasound-guided coracohumeral ligament release. Orthop Traumatol Surg Res. 2018; 104(6):823–827. PMID:
29567320.

28. Hagiwara Y, Ando A, Kanazawa K, Koide M, Sekiguchi T, Hamada J, et al. Arthroscopic coracohumeral ligament release for patients with frozen shoulder. Arthrosc Tech. 2017; 7(1):e1–5. PMID:
29379707.

29. DePalma AF. The classic. Loss of scapulohumeral motion (frozen shoulder). Ann Surg. 1952;135:193-204. Clin Orthop Relat Res. 2008; 466(3):552–560. PMID:
18264843.
30. Leffert RD. The frozen shoulder. Instr Course Lect. 1985; 34:199–203. PMID:
3833940.
31. Ozaki J, Nakagawa Y, Sakurai G, Tamai S. Recalcitrant chronic adhesive capsulitis of the shoulder. Role of contracture of the coracohumeral ligament and rotator interval in pathogenesis and treatment. J Bone Joint Surg Am. 1989; 71(10):1511–1515. PMID:
2592391.

32. Tetro AM, Bauer G, Hollstien SB, Yamaguchi K. Arthroscopic release of the rotator interval and coracohumeral ligament: an anatomic study in cadavers. Arthroscopy. 2002; 18(2):145–150. PMID:
11830807.
33. Mengiardi B, Pfirrmann CW, Gerber C, Hodler J, Zanetti M. Frozen shoulder: MR arthrographic findings. Radiology. 2004; 233(2):486–492. PMID:
15358849.

34. Zhao W, Zheng X, Liu Y, Yang W, Amirbekian V, Diaz LE, et al. An MRI study of symptomatic adhesive capsulitis. PLoS One. 2012; 7(10):e47277. PMID:
23082152.

35. Lee SY, Park J, Song SW. Correlation of MR arthrographic findings and range of shoulder motions in patients with frozen shoulder. AJR Am J Roentgenol. 2012; 198(1):173–179. PMID:
22194494.

36. Yang HF, Tang KL, Chen W, Dong SW, Jin T, Gong JC, et al. An anatomic and histologic study of the coracohumeral ligament. J Shoulder Elbow Surg. 2009; 18(2):305–310. PMID:
19095467.

37. Toprak U, Ustüner E, Uyanık S, Aktaş G, Kınıklı GI, Baltacı G, et al. Comparison of ultrasonographic patellar tendon evaluation methods in elite junior female volleyball players: thickness versus cross-sectional area. Diagn Interv Radiol. 2012; 18(2):200–207. PMID:
22105710.
38. Bang YS, Park J, Lee SY, Park J, Park S, Joo Y, et al. Value of anterior band of the inferior glenohumeral ligament area as a morphological parameter of adhesive capsulitis. Pain Res Manag. 2019; 2019:9301970. PMID:
31205575.

39. Couppé C, Svensson RB, Sødring-Elbrønd V, Hansen P, Kjaer M, Magnusson SP. Accuracy of MRI technique in measuring tendon cross-sectional area. Clin Physiol Funct Imaging. 2014; 34(3):237–241. PMID:
24119143.

40. Galanis N, Savvidis M, Tsifountoudis I, Gkouvas G, Alafropatis I, Kirkos J, et al. Correlation between semitendinosus and gracilis tendon cross-sectional area determined using ultrasound, magnetic resonance imaging and intraoperative tendon measurements. J Electromyogr Kinesiol. 2016; 26:44–51. PMID:
26708406.

41. Cho H, Kang S, Won HS, Yang M, Kim YD. New insights into pathways of the dorsal scapular nerve and artery for selective dorsal scapular nerve blockade. Korean J Pain. 2019; 32(4):307–312. PMID:
31569924.

42. Ghavidel-Parsa B, Bidari A, Hajiabbasi A, Shenavar I, Ghalehbaghi B, Sanaei O. Fibromyalgia diagnostic model derived from combination of American College of Rheumatology 1990 and 2011 criteria. Korean J Pain. 2019; 32(2):120–128. PMID:
31091511.

43. Kim YD, Yu JY, Shim J, Heo HJ, Kim H. Risk of encountering dorsal scapular and long thoracic nerves during ultrasound-guided interscalene brachial plexus block with nerve stimulator. Korean J Pain. 2016; 29(3):179–184. PMID:
27413483.

44. Schwenk ES, Mariano ER. Designing the ideal perioperative pain management plan starts with multimodal analgesia. Korean J Anesthesiol. 2018; 71(5):345–352. PMID:
30139215.

45. Shanmugam S, Mathias L, Thakur A, Kumar D. Effects of intramuscular electrical stimulation using inversely placed electrodes on myofascial pain syndrome in the shoulder: a case series. Korean J Pain. 2016; 29(2):136–140. PMID:
27103970.

46. Tantawy SA, Abdul Rahman A, Abdul Ameer M. The relationship between the development of musculoskeletal disorders, body mass index, and academic stress in Bahraini University students. Korean J Pain. 2017; 30(2):126–133. PMID:
28416996.

47. Lee S, Cho HR, Yoo JS, Kim YU. The prognostic value of median nerve thickness in diagnosing carpal tunnel syndrome using magnetic resonance imaging: a pilot study. Korean J Pain. 2020; 33(1):54–59. PMID:
31888318.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download