INTRODUCTION
Gout is one of a heterogeneous group of diseases related to hyperuricemia. The causes of uric acid elevation can be divided into either excessive uric acid production or decreased uric acid excretion. Xanthine oxidase inhibitors that block uric acid production are the usual first-choice of urate-lowering therapy,
12 but uricosuric drugs, such as probenecid, benzbromarone and sulfinpyrazone, are also effective at reducing serum uric acid levels. However, uricosuric agents can be used in patients with good renal function who excrete uric acid of < 600 mg/day,
3 because higher concentrations of uric acid in the collecting tubules can predispose to uric acid stone formation.
4 The most effective method to measure the amount of urinary uric acid excretion is the 24-hour urine collection. However, there are several disadvantages in 24-hour urine collection, that is, it is cumbersome, inconvenient, and sometimes unreliable because of errors in sample collection. Therefore, the authors investigated the efficacy of the random urine uric acid-to-creatinine (UA/CR) ratio, as a simpler alternative to the 24-hour urine collection, to screen the patients with gout who excrete 24-hour urine uric acid less than 600 mg/day.
Go to :

DISCUSSION
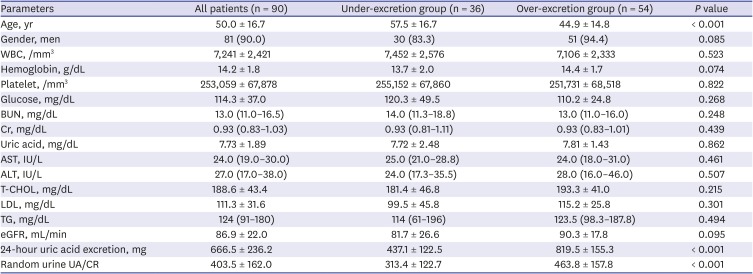
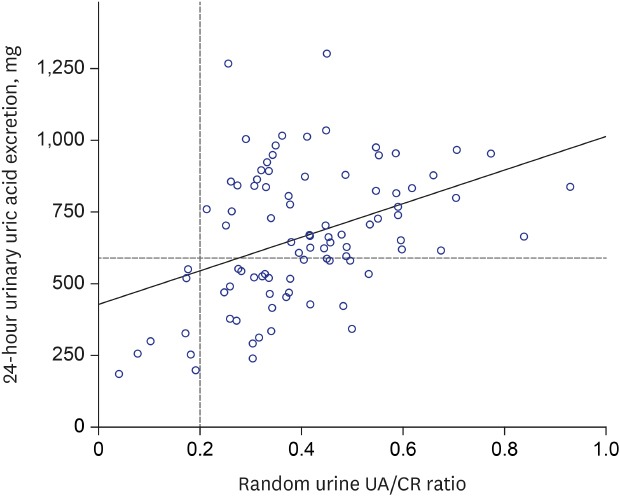
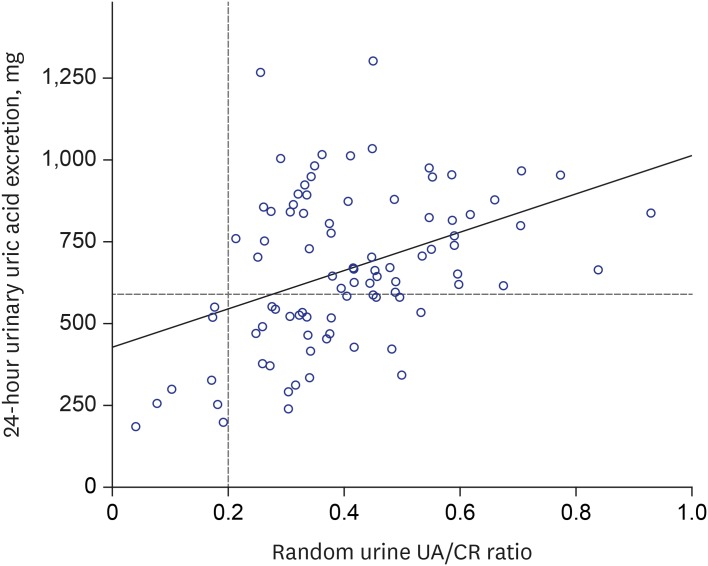
In our study, we found a moderate correlation between the random urine UA/CR ratio and 24-hour urine uric acid excretion. We detected the uric acid under-excretion group, with a cut-off value of urine UA/CR ratio 0.342. However, the random UA/CR less than 0.2 detected all the patients less than 600 mg uric acid in 24-hour urine collection (the positive predictive value, 100%). Therefore, this study suggests that UA/CR 0.2 would be a useful predictor to screen gouty patients who can be treated with uricosuric drugs.
There has been one previous study comparing the spot UA/CR ratio with 24-hour urine uric acid excretion in patients with gout and it evaluated whether the spot UA/CR ratio is useful for the estimation of urine uric acid over-excretion.
7 Moriwaki et al.
7 showed a statistically significant correlation between 24-hour urine uric acid excretion and the spot urine UA/CR ratio. They defined over-excretion as above 1,000 mg/day, and the cutoff value was defined as a mean + 2 SD. The cutoff value of the spot urine UA/CR ratio was 0.63, and the diagnostic accuracy of uric acid over-excretion was 87.2%, but the sensitivity was 25.0%. The sensitivity was so low in that study that the possibility of uric acid over-excretion could not be ruled out. Therefore, it was concluded the UA/CR ratio using spot urine was not an accurate indicator of uric acid over-excretion in patients with gout.
That previous study had similar findings to our study. Our study showed the random urinary UA/CR ratio correlated with the 24-hour urine uric acid excretion. Furthermore, the correlation between them was similar in CKD patients with a GFR < 60 mL/min/1.73 m
2. There are many cases of decreased renal function among patients with gout, so that this result suggests that the random urine UA/CR ratio may be useful even for CKD patients. In contrast, our study defined uric acid over-excretion as 600 mg/day. There are various opinions about the standard of uric acid over excretion from 600 mg to 700 mg, 800 mg and 1,000 mg.
3789 There is not the exact standard of uric acid over excretion. The paper of Perez-Ruiz et al.
8 showed that mean and median uric acid excretion in healthy subjects for 24 hours were 594 ± 143 mg, and 605 mg/day/1.73 m
2, respectively. However, any authors of the above papers including the Harrison's textbook did not explain how and why to set those values as the standards for uric acid over-excretion in detail.
3789 In the present study, the authors looked for a minimal cut-off value that could safely use the uricosuric agent. Therefore, the research was carried out based on 600 mg, the lowest risk of uric acid stone formation.
Recently, there has been a study on the correlation between 24-hour urine uric acid excretion and UA/CR ratio in random and morning urine for individuals regardless of gout.
10 In that study, the correlation coefficients were 0.193 in random urine and 0.219 in morning urine, which were slightly lower than the result of our study.
The random urine UA/CR ratio showed positive correlation with 24-hour uric acid excretion, but less correlation compared to the results of the random urine protein-to-Cr ratio and 24-hour urine protein excretion.
111213 One study showed the correlation coefficient (
r) was 0.9 between the random urine protein-to-Cr ratio and 24-hour urine protein excretion.
12
A question emerges as to why the correlation coefficient of our study was relatively lower than that of the study on the urine protein-to-Cr ratio, for which there are several possible answers. First, there is the difference in the mechanism in which uric acid and protein are excreted in the kidney. Renal excretion of uric acid represents approximately 60% to 70% of total uric acid excretion from the body.
14 A smaller proportion of uric acid is secreted in the intestine and is further metabolized by resident gut bacteria.
14 Most uric acid is freely filtered in the kidney, and tubular secretion and reabsorption coexist along the entire length of the proximal tubule.
15 Roughly 90% of the filtered load is normally reabsorbed along the nephron, so that the fractional excretion of uric acid averages 5% to 10%.
16 URAT1 and GLUT5 are considered as the main pathways of urate reabsorption, and ABCG2 is the major efflux pathway on the apical side.
17 Drugs such as probenecid and losartan inhibit URAT1, accounting for their uricosuric effect.
1819 In contrast, most of the proteins, especially albumin, that are filtered from the kidney are excreted in the urine.
Additionally, the range of daily proteinuria was very wide, ranging from 0.03 to 10 g. However, the range of daily urinary uric acid excretion was approximately 0.02 to 1.5 g. As the range of uric acid excretion was narrower compared with the proteinuria range, a small error in the measured value may also have caused a statistical difference.
Our study was performed as a retrospective study, so we could not collect the urine samples at the same time of the day, unfortunately. We did not attempt to limit the time to collect random urine, or food consumption or the ordinary activities of daily life. Several factors affect urinary uric acid concentration, including circadian rhythm, eating habits, and physical activity.
720 The highest uric acid concentration in healthy participants has been reported between 5 a.m. and 8 a.m. and the lowest between 11 p.m. and 2 a.m.
20 We did not attempt to limit the time to collect random urine, or food consumption or the ordinary activities of daily life. This is a weak point of this study. The second limitation of this study is the lack of healthy control. However, it is known that there is a difference in uric acid excretion between healthy and gouty patients.
821 Therefore, this study was focused on the patients with gout and was intended to evaluate whether the random urine UA/CR ratio could be helpful for the screening in patients with gout planning to use uricosuric agents. Finally, our study involved a small population. If more patients had been enrolled, more accurate results might have been obtained. Therefore, further research needs to be done involving more patients.
In conclusion, the random urine UA/CR ratio weakly correlated with 24-hour urine uric acid excretions in patients with gout. However, the random urine UA/CR ratio 0.2 would be a useful predictor to screen the gouty patients who excrete 24-hour urine uric acid less than 600 mg/day, so that, need to be treated with uricosuric drugs.
Go to :





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download