Abstract
Botulism is a rare neuromuscular disorder caused by neurotoxins produced by Clostridium botulinum. The diagnosis of infant botulism may be obscured or delayed, as its presentation is similar to that of infantile neuromuscular disorders. We report the first Korean case of infant botulism in an acute progressive floppy infant with poor sucking and a weak cry. No abnormalities were found in all blood, cerebrospinal fluid, genetic test, nerve conduction study, and imaging studies. A stool-toxin test was finally performed under suspicion of infant botulism, and the result was positive. The patient was immediately treated with heptavalent botulism antitoxin. Follow-up after 3 months showed normal development with a complete resolution of all symptoms. Therefore, clinical suspicion of infant botulism, which is a treatable infantile neuromuscular disease, is essential for early diagnosis and prompt treatment in the differential diagnosis of a floppy infant.
Go to : 
Graphical Abstract
Go to : 
Botulism is a flaccid nerve paralysis disease caused by Clostridium botulinum, whose spores produce botulinum—a neurotoxin that blocks acetylcholine release at neuronal endings.1 Infant botulism occurs when ingested C. botulinum spores germinate and colonize the infant's gastrointestinal tract.2 Its typical clinical symptoms include constipation, hyporeflexia, hypotonia, cranial nerve disorders, and respiratory difficulties.23 However, these symptoms are difficult to distinguish from other infantile neuromuscular diseases such as Guillain-Barre syndrome, spinal muscular atrophy, myasthenia gravis, and neurometabolic diseases. Although reported from other countries, infantile botulism has not yet been reported in Korea. Botulism is often ignored during the differential diagnosis of a floppy infant due to its rarity and vague symptoms, and insufficient evidence for exposure to C. botulinum spores. We report the first Korean case of infant botulism successfully treated with heptavalent botulism antitoxin, along with its characteristic electromyography clues.
Go to : 
On 7 June 2019, a previously healthy, 4-month-old female infant was admitted with an initial impression of gastrointestinal sepsis, 7 days after developing symptoms including lethargy, decreased oral intake, and a weak cry. Laboratory investigation revealed normal blood gas analysis, serum electrolytes, erythrocyte sedimentation rate, renal function tests, and C-reactive protein levels. All cultures (blood, urine, stool, and cerebrospinal fluid) were negative, and the abdominal ultrasound and oropharyngoscopy were normal. The patient's condition worsened despite the administration of intravenous fluids and antibiotics.
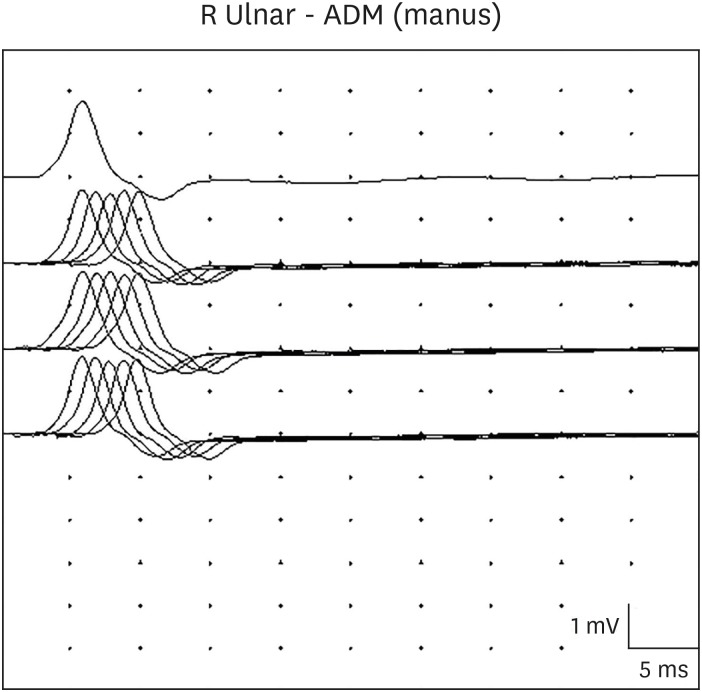
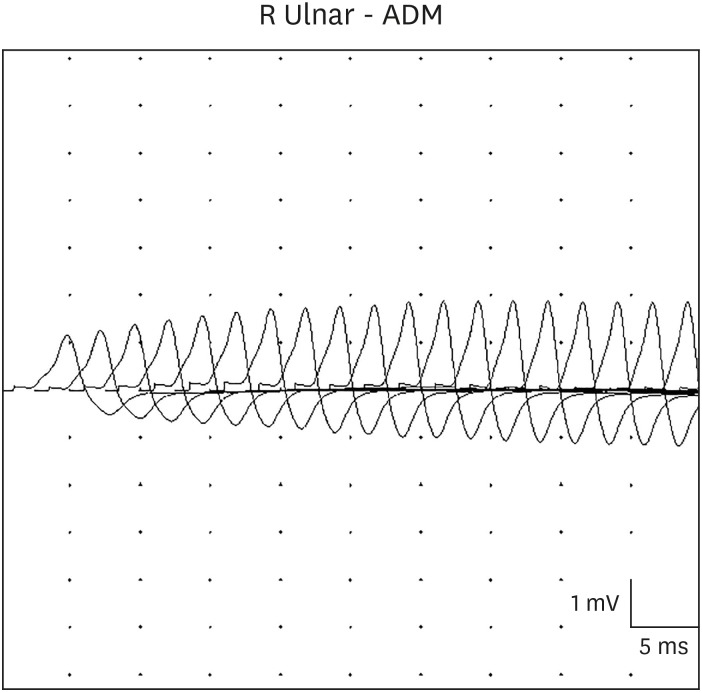
On hospital day 3, a neurology consultation revealed global hypotonia with loss of head control, and minimal response to pain. The patient had no spontaneous eye movements, ptosis, a poor gag reflex, and a weak cry. The deep tendon reflexes were absent. Extensive neurological studies were conducted to rule out encephalitis, Guillain-Barre syndrome, spinal muscular atrophy, myasthenia gravis, and neurometabolic diseases. The brain and spine magnetic resonance imagings, and the electroencephalography were normal. The anti-acetylcholine receptor antibody test, nerve conduction test, and the genetic tests for spinal muscular atrophy and mitochondrial disorders were negative. The metabolic workup, including serum amino acid and urine organic acid analysis, was normal. A videofluoroscopic swallowing study showed both tracheal penetration and aspiration, with no efforts to eject the bolus. Repetitive motor nerve stimulation showed low compound muscle action potential amplitude of the right abductor digiti minimi muscle, and no decrement at low frequencies (2, 3, and 5 Hz) (Fig. 1), but a significant incremental response at high frequency (50 Hz) (Fig. 2). These findings suggested a presynaptic neuromuscular junction disorder such as botulism. On hospital day 10, serum and stool specimens were transferred to the Korea Centers for Disease Control and Prevention for a mouse toxin neutralization test, under suspicion of infantile botulism. The serum test was negative, but the stool test was positive for toxins A and B. The Korea Centers for Disease Control and Prevention inspected the patient's powdered milk, cleaner dust, and water in the water purifier. However, the source of spore contamination could not be identified. We immediately treated with heptavalent botulism antitoxin after a confirmed diagnosis of infant botulism. The patient tolerated heptavalent botulism antitoxin without any hypersensitivity reactions or serum sickness. Three weeks after heptavalent botulism antitoxin treatment and exercise rehabilitation, both oral intake and muscle tone showed improvement. After one month, a videofluoroscopic swallowing study showed no tracheal penetration or aspiration during the swallowing of fluids. The total hospital period was 40 days, with nasogastric tube feeding for 34 days. At follow-up in the neurology clinic 3 months later, she showed normal development and complete resolution of all symptoms.
This case was approved by the Institutional Review Board (IRB) of Ajou University Hospital (IRB No. MED-EXP-19-551) and the IRB waived informed consent for this reporting.
Go to : 
Infant botulism is caused by botulinum—the potent neurotoxin produced by C. botulinum spores in the gut. Botulinum binds to presynaptic cholinergic receptors at motor nerve terminals, is subsequently internalized, and behaves as a protease—damaging an integral membrane protein of acetylcholine-containing vesicles, and inhibiting the release of acetylcholine necessary for muscle excitation.2 Botulism has been recognized on all continents except Africa.4 The infant form is the most common in the United States. This report describes the first Korean case of infant botulism, caused by C. botulinum type A and B toxins. Since the infection occurs in the gastrointestinal tract, many foods consumed by infants have been investigated by various researchers. The most potentially recognized sources of C. botulinum spores are dust and honey.567 In many cases of infant botulism, contaminated honey consumption has been implicated. However, it is worth noting that in most cases, the definitive source of C. botulinum spores has not been identified.8 Investigators have also frequently noted environmental conditions that might expose infants directly to sources of C. botulinum spores. The organism causing infant botulism is usually found in the soil of the area where the illness occurs.9 In Australia and Japan, infant botulism has been associated with the C. botulinum spores found in yard soils, vacuum cleaner dust, and drinking water.1011 Although honey is the only recognized and avoidable food source directly associated with infant botulism, in our case, the patient did not have a history of honey consumption. Moreover, the Korea Centers for Disease Control and Prevention could not identify the source of spore contamination in the environment of the patient's house or food products.
The clinical spectrum of infant botulism varies considerably from mild cases to severe and fulminant cases that lead to sudden infant death.1213 Its symptoms include a weak cry, an impaired gag reflex, poor sucking ability, decreased oral intake, loss of head control, and generalized weakness with cranial nerve involvement. Loss of ocular movement, mydriasis, and ptosis may also occur.214 However, these symptoms are often initially absent and are frequently replaced by poorly defined symptoms, including poor sucking and feeding difficulties, which may be confused with gastrointestinal symptoms, as in our case. Medical personnel should be aware that symptoms such as poor sucking or weak crying may occur as a result of bulbar weakness. The most similar diagnoses to infant botulism are spinal muscular atrophy (type I) and Guillain-Barre syndrome.14 However, spinal muscular atrophy (type I) patients typically show a longer history of hypotonia, while infant botulism patients exhibit subacute to acute hypotonia during disease onset. Although loss of ocular movements and ptosis occur in both Miller Fisher variant of Guillain-Barre syndrome and infant botulism, these diagnoses may be differentiated by nerve conduction studies, electromyography, and cerebrospinal fluid analysis.15 The patient's age can also help with differentiation; 95% of infant botulism occurs in patients < 6 months of age, on the other hand, Guillain-Barre syndrome generally occurs in older patients.15
In most infant botulism, electromyography reveals a characteristic, but not diagnostic, pattern of brief-duration, small-amplitude, and abundant motor-unit action potentials, and an incremental response to rapid repetitive nerve stimulation at high frequencies, as shown in our case.16 These findings can suggest myopathy, but in infant botulism, they are due to the functional denervation of myofibers due to impairment of presynaptic acetylcholine release. A diminishing response is possible at 2 Hz repetitive stimulation, but is not a distinguishing feature, and could also indicate myasthenic syndromes. Although nerve conduction studies are typically normal in botulism, technical difficulties can occur, and a normal electromyography does not rule out botulism. Laboratory criteria for the diagnosis of infant botulism include the isolation of C. botulinum from the stool, or the detection of botulinum in the stool or serum. Since the level of circulating toxins is low, the detection of toxins in serum is often negative and inconclusive. A positive serum result may help diagnoses, but a negative serum result does not rule out infant botulism.15 The only reliable confirmatory test for infant botulism is a toxin neutralization mouse bioassay on the stool filtrate.17 In our case, the patient's serum test and stool culture were negative, but the stool toxin test was positive.
Human-derived botulism immune globulin intravenous (BIG-IV) neutralizes the free botulinum toxin, and is approved in the United States as a treatment for infant botulism by type A or B toxins in patients below 1 year of age.18 While BIG-IV does not improve the symptoms immediately, it accelerates clinical improvement by preventing botulinum from binding further. Because earlier treatment with BIG-IV shortens the duration of hospitalization, the decision of treatment should be carried out according to the clinical diagnosis of botulism, without delay for a confirmatory laboratory test.13 In order to receive BabyBIG® (BIG-IV), physicians should contact the infant botulism treatment and prevention program (Division of Communicable Disease Control, California Department of Public Health). The current cost for BabyBIG® is USD 57,300. This is a flat fee regardless of the number of vials shipped to treat the patient and does not include the cost for a hospital stay. Due to its relatively high cost and transit time, it was not given to our patient. Horse plasma-derived heptavalent botulism antitoxin—a mixture of antibody fragments that neutralizes the botulism-causing neurotoxin (A-G) serotypes—was licensed in 2013 to treat patients suspected of botulism. Heptavalent botulism antitoxin is the only product available for the treatment of botulism in Korea, with the most common side effects including fever, headache, chills, itching, rash, and nausea. Because heptavalent botulism antitoxin is manufactured from horse plasma, it may also cause allergic reactions or a delayed hypersensitivity reaction (serum sickness) in people sensitive to horse proteins, and vulnerable pediatric patients.19 Heptavalent botulism antitoxin (Emergent BioSolution Canada Inc., Toronto, ON, Canada) is intended for slow intravenous infusion after a 1:10 dilution in normal saline and at the following dose: infants (< 1 year) are prescribed 10% of the adult dose (one vial) regardless of body weight (each dose of 50 mL is equivalent to one adult dose). A starting infusion rate (for the first 30 minutes) of 0.01 mL/kg/min was used with an incremental increase every 30 minutes, if tolerated, of 0.01 mL/kg/min up to a maximum of 0.03 mL/kg/min. Our patient tolerated heptavalent botulism antitoxin, and did not develop any hypersensitivity reactions or serum sickness.
In conclusion, the symptoms of infant botulism are difficult to distinguish from those of other infantile neuromuscular diseases. Electromyography—characterized by incremental responses to high frequency repetitive nerve stimulation—is useful for the diagnosis. For rapid diagnosis and prompt treatment, early clinical suspicion of infant botulism is essential in the differential diagnosis of a floppy infant.
Go to : 
ACKNOWLEDGMENTS
The authors thank the Korea Centers for Disease Control and Prevention for providing the patient with heptavalent botulism antitoxin for free.
Go to : 
References
1. Lund BM. Foodborne disease due to Bacillus and Clostridium species. Lancet. 1990; 336(8721):982–986. PMID: 1977014.

2. Cox N, Hinkle R. Infant botulism. Am Fam Physician. 2002; 65(7):1388–1392. PMID: 11996423.
3. Schreiner MS, Field E, Ruddy R. Infant botulism: a review of 12 years' experience at the Children's Hospital of Philadelphia. Pediatrics. 1991; 87(2):159–165. PMID: 1898989.
4. Koepke R, Sobel J, Arnon SS. Global occurrence of infant botulism, 1976–2006. Pediatrics. 2008; 122(1):e73–82. PMID: 18595978.

5. Kauiter DA, Lilly T Jr, Solomon HM, Lynt RK. Clostridium botulinum spores in infant foods: a survey. J Food Prot. 1982; 45(11):1028–1029. PMID: 30913614.
6. Thompson JA, Glasgow LA, Warpinski JR, Olson C. Infant botulism: clinical spectrum and epidemiology. Pediatrics. 1980; 66(6):936–942. PMID: 7005856.
7. Hauschild AH, Hilsheimer R, Weiss KF, Burke RB. Clostridium botulinum in honey, syrups and dry infant cereals. J Food Prot. 1988; 51(11):892–894. PMID: 30991497.
8. Rosow LK, Strober JB. Infant botulism: review and clinical update. Pediatr Neurol. 2015; 52(5):487–492. PMID: 25882077.

9. Long SS, Gajewski JL, Brown LW, Gilligan PH. Clinical, laboratory, and environmental features of infant botulism in Southeastern Pennsylvania. Pediatrics. 1985; 75(5):935–941. PMID: 3887319.
10. Murrell WG, Stewart BJ. Botulism in New South Wales, 1980–1981. Med J Aust. 1983; 1(1):13–17. PMID: 6400580.

11. Takahashi M, Shimizu T, Ooi K, Noda H, Nasu T, Sakaguchi G. Quantification of Clostridium botulinum type A toxin and organisms in the feces of a case of infant botulism and examination of other related specimens. Jpn J Med Sci Biol. 1988; 41(1):21–26. PMID: 3057266.
12. Mitchell WG, Tseng-Ong L. Catastrophic presentation of infant botulism may obscure or delay diagnosis. Pediatrics. 2005; 116(3):e436–8. PMID: 16140690.

13. Nevas M, Lindström M, Virtanen A, Hielm S, Kuusi M, Arnon SS, et al. Infant botulism acquired from household dust presenting as sudden infant death syndrome. J Clin Microbiol. 2005; 43(1):511–513. PMID: 15635031.

14. Wigginton JM, Thill P. Infant botulism. A review of the literature. Clin Pediatr (Phila). 1993; 32(11):669–674. PMID: 8299297.
15. Fenicia L, Anniballi F. Infant botulism. Ann Ist Super Sanita. 2009; 45(2):134–146. PMID: 19636165.
16. Cherington M. Electrophysiologic methods as an aid in diagnosis of botulism: a review. Muscle Nerve. 1982; 5(9S):S28–9. PMID: 6763148.
17. Rao AK, Lin NH, Griese SE, Chatham-Stephens K, Badell ML, Sobel J. Clinical criteria to trigger suspicion for botulism: an evidence-based tool to facilitate timely recognition of suspected cases during sporadic events and outbreaks. Clin Infect Dis. 2017; 66(suppl_1):S38–S42. PMID: 29293926.

18. Arnon SS. Creation and development of the public service orphan drug Human Botulism Immune Globulin. Pediatrics. 2007; 119(4):785–789. PMID: 17403850.

19. Yu PA, Lin NH, Mahon BE, Sobel J, Yu Y, Mody RK, et al. Safety and improved clinical outcomes in patients treated with new equine-derived heptavalent botulinum antitoxin. Clin Infect Dis. 2017; 66(suppl_1):S57–S64. PMID: 29293928.

Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download