This article has been
cited by other articles in ScienceCentral.
Abstract
The impact of bronchiectasis on the occurrence of postoperative pulmonary complications (PPC) after extra-pulmonary surgery in patients with airflow limitation is not well elucidated. A retrospective analysis of 437 patients with airflow limitations, including 62 patients with bronchiectasis, was conducted. The analysis revealed that bronchiectasis was associated with increased PPC (adjusted odds ratio [aOR], 2.73; P = 0.001), which was especially significant in patients who did not use bronchodilators (aOR, 3.24; P = 0.002). Our study indicates that bronchiectasis is associated with an increased risk of PPC following extra-pulmonary surgery in patients with airflow limitation, and bronchodilators may prevent PPC in these patients.
Keywords: Bronchiectasis, Spirometry, Complications, Surgery
Bronchiectasis has been regarded as an orphan lung disease; however, the burden imposed by this disease is substantial in many countries, including Korea.
1 Patients with bronchiectasis often have coexisting airflow limitation, which is associated with more respiratory infections and exacerbations.
23
The presence of an airflow limitation is a well-defined risk factor for postoperative pulmonary complications (PPC) in patients undergoing extra-pulmonary surgery,
4 leading to increased in-hospital mortality and prolonged hospital stay.
5 However, limited data are available to clarify whether bronchiectasis further increases PPC in patients with airflow limitation. The aim of the present study was to investigate the impact of bronchiectasis on PPC following extra-pulmonary surgery in patients with airflow limitation.
We reviewed the medical records of 1,891 adult patients referred to the pulmonary department for consultation before extra-thoracic surgery at Samsung Medical Center (a 1,979-bed referral hospital) between March 2014 and January 2015. Of these, we included 440 who showed airflow limitation (i.e., pre-bronchodilator forced expiratory volume in 1 second/forced vital capacity < 0.7) on spirometry and had available chest computed tomography (CT) data. Three patients with asthma were not included in this study. Finally, 437 patients were included in this study. We classified the patients into two groups according to the bronchiectasis on chest CT and observed the occurrence of PPC over time. Some clinical data of the patients were included in a recently published article.
4
PPC was defined as in-hospital postoperative events related to the respiratory system, such as respiratory infection, respiratory failure, pleural effusion, atelectasis, pneumothorax, and bronchospasm (post-operative exacerbation) within the first seven postoperative days.
6 We also extracted preoperative data, including information regarding demographic characteristics, body mass index, smoking history, American Society of Anesthesiologists physical status, comorbidities, types of surgery, pulmonary function test, and bronchodilator use. The severity of bronchiectasis was assessed by the Bronchiectasis Severity Index (BSI).
7 We designated 0–4 points and ≥ 5 points as bronchiectasis of low severity and bronchiectasis of high severity, respectively.
7
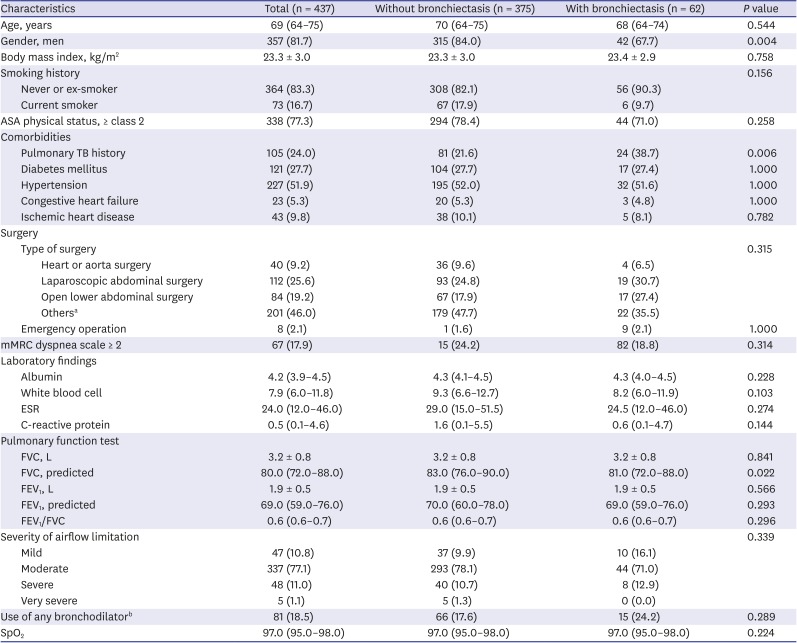
Of the patients, 62 (14.2%) had bronchiectasis. Women gender (32.3% vs. 16.0%,
P = 0.004) and previous history of tuberculosis (38.7% vs. 21.6%,
P = 0.006) were more common in patients with bronchiectasis compared to those without bronchiectasis. There were no significant intergroup differences in age, smoking history, type of surgery, pulmonary function test results, bronchodilator use, and severity of airflow limitation (
Table 1). The median number of involved lobes was two (interquartile range, 1–3). The main type of bronchiectasis was cystic (48.4%) followed by cylindrical (30.6%) and mixed (21.0%).
Table 1
Baseline characteristics of the study population
|
Characteristics |
Total (n = 437) |
Without bronchiectasis (n = 375) |
With bronchiectasis (n = 62) |
P value |
|
Age, years |
69 (64–75) |
70 (64–75) |
68 (64–74) |
0.544 |
|
Gender, men |
357 (81.7) |
315 (84.0) |
42 (67.7) |
0.004 |
|
Body mass index, kg/m2
|
23.3 ± 3.0 |
23.3 ± 3.0 |
23.4 ± 2.9 |
0.758 |
|
Smoking history |
|
|
|
0.156 |
|
Never or ex-smoker |
364 (83.3) |
308 (82.1) |
56 (90.3) |
|
Current smoker |
73 (16.7) |
67 (17.9) |
6 (9.7) |
|
ASA physical status, ≥ class 2 |
338 (77.3) |
294 (78.4) |
44 (71.0) |
0.258 |
|
Comorbidities |
|
|
|
|
|
Pulmonary TB history |
105 (24.0) |
81 (21.6) |
24 (38.7) |
0.006 |
|
Diabetes mellitus |
121 (27.7) |
104 (27.7) |
17 (27.4) |
1.000 |
|
Hypertension |
227 (51.9) |
195 (52.0) |
32 (51.6) |
1.000 |
|
Congestive heart failure |
23 (5.3) |
20 (5.3) |
3 (4.8) |
1.000 |
|
Ischemic heart disease |
43 (9.8) |
38 (10.1) |
5 (8.1) |
0.782 |
|
Surgery |
|
|
|
|
|
Type of surgery |
|
|
|
0.315 |
|
|
Heart or aorta surgery |
40 (9.2) |
36 (9.6) |
4 (6.5) |
|
|
Laparoscopic abdominal surgery |
112 (25.6) |
93 (24.8) |
19 (30.7) |
|
|
Open lower abdominal surgery |
84 (19.2) |
67 (17.9) |
17 (27.4) |
|
|
Othersa
|
201 (46.0) |
179 (47.7) |
22 (35.5) |
|
Emergency operation |
8 (2.1) |
1 (1.6) |
9 (2.1) |
1.000 |
|
mMRC dyspnea scale ≥ 2 |
67 (17.9) |
15 (24.2) |
82 (18.8) |
0.314 |
|
Laboratory findings |
|
|
|
|
|
Albumin |
4.2 (3.9–4.5) |
4.3 (4.1–4.5) |
4.3 (4.0–4.5) |
0.228 |
|
White blood cell |
7.9 (6.0–11.8) |
9.3 (6.6–12.7) |
8.2 (6.0–11.9) |
0.103 |
|
ESR |
24.0 (12.0–46.0) |
29.0 (15.0–51.5) |
24.5 (12.0–46.0) |
0.274 |
|
C-reactive protein |
0.5 (0.1–4.6) |
1.6 (0.1–5.5) |
0.6 (0.1–4.7) |
0.144 |
|
Pulmonary function test |
|
|
|
|
|
FVC, L |
3.2 ± 0.8 |
3.2 ± 0.8 |
3.2 ± 0.8 |
0.841 |
|
FVC, predicted |
80.0 (72.0–88.0) |
83.0 (76.0–90.0) |
81.0 (72.0–88.0) |
0.022 |
|
FEV1, L |
1.9 ± 0.5 |
1.9 ± 0.5 |
1.9 ± 0.5 |
0.566 |
|
FEV1, predicted |
69.0 (59.0–76.0) |
70.0 (60.0–78.0) |
69.0 (59.0–76.0) |
0.293 |
|
FEV1/FVC |
0.6 (0.6–0.7) |
0.6 (0.6–0.7) |
0.6 (0.6–0.7) |
0.296 |
|
Severity of airflow limitation |
|
|
|
0.339 |
|
Mild |
47 (10.8) |
37 (9.9) |
10 (16.1) |
|
Moderate |
337 (77.1) |
293 (78.1) |
44 (71.0) |
|
Severe |
48 (11.0) |
40 (10.7) |
8 (12.9) |
|
Very severe |
5 (1.1) |
5 (1.3) |
0 (0.0) |
|
Use of any bronchodilatorb
|
81 (18.5) |
66 (17.6) |
15 (24.2) |
0.289 |
|
SpO2
|
97.0 (95.0–98.0) |
97.0 (95.0–98.0) |
97.0 (95.0–98.0) |
0.224 |

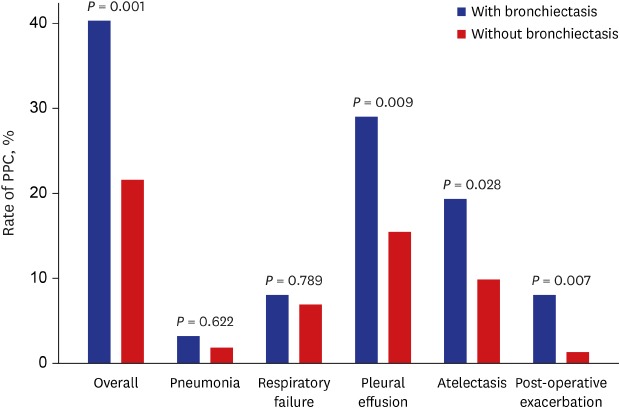
The overall rate of PPC was significantly higher in patients with bronchiectasis than in those without bronchiectasis (40.3% vs. 21.6%,
P = 0.001). The occurrence of pleural effusion (29.0% vs. 15.5%,
P = 0.009), atelectasis (19.4% vs. 9.9%,
P = 0.028), and post-operative exacerbation (8.1% vs. 1.3%,
P = 0.007) was significantly higher in patients with bronchiectasis than in those without bronchiectasis, while there were no significant differences in the occurrence of pneumonia and respiratory failure between the two groups (
Fig. 1). The rate of PPC by bronchiectasis and BSI categories are shown in
Supplementary Fig. 1.
Fig. 1
The rate of PPC following extra-pulmonary surgery in patients with airflow limitation according to the presence or absence of bronchiectasis.
PPC = postoperative pulmonary complications.

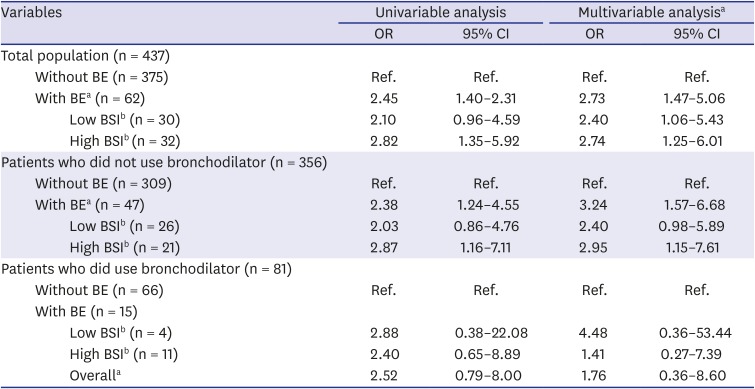
As shown in
Table 2, bronchiectasis was associated with the occurrence of PPC (adjusted odds ratio [aOR] for PPC, 2.73; 95% confidence interval [CI], 1.47–5.06). Compared to patients without bronchiectasis, those showing bronchiectasis with low and high BSI were 2.40 (95% CI, 1.06–5.43) and 2.74 (95% CI, 1.25–6.01) fold more likely to have PPC after extra-pulmonary surgery, respectively (
Table 2).
Table 2
Unadjusted and adjusted ORs for the occurrence of PPC
|
Variables |
Univariable analysis |
Multivariable analysisa
|
|
OR |
95% CI |
OR |
95% CI |
|
Total population (n = 437) |
|
|
|
|
|
Without BE (n = 375) |
Ref. |
Ref. |
Ref. |
Ref. |
|
With BEa (n = 62) |
2.45 |
1.40–2.31 |
2.73 |
1.47–5.06 |
|
|
Low BSIb (n = 30) |
2.10 |
0.96–4.59 |
2.40 |
1.06–5.43 |
|
|
High BSIb (n = 32) |
2.82 |
1.35–5.92 |
2.74 |
1.25–6.01 |
|
Patients who did not use bronchodilator (n = 356) |
|
|
|
|
|
Without BE (n = 309) |
Ref. |
Ref. |
Ref. |
Ref. |
|
With BEa (n = 47) |
2.38 |
1.24–4.55 |
3.24 |
1.57–6.68 |
|
|
Low BSIb (n = 26) |
2.03 |
0.86–4.76 |
2.40 |
0.98–5.89 |
|
|
High BSIb (n = 21) |
2.87 |
1.16–7.11 |
2.95 |
1.15–7.61 |
|
Patients who did use bronchodilator (n = 81) |
|
|
|
|
|
Without BE (n = 66) |
Ref. |
Ref. |
Ref. |
Ref. |
|
With BE (n = 15) |
|
|
|
|
|
|
Low BSIb (n = 4) |
2.88 |
0.38–22.08 |
4.48 |
0.36–53.44 |
|
|
High BSIb (n = 11) |
2.40 |
0.65–8.89 |
1.41 |
0.27–7.39 |
|
|
Overalla
|
2.52 |
0.79–8.00 |
1.76 |
0.36–8.60 |

The ORs for PPC significantly differed with bronchodilator use; among patients who did use bronchodilators, the OR for PPC was not significantly higher in patients with bronchiectasis in comparison with that in patients without bronchiectasis. In contrast, among patients who did not use bronchodilators, the OR for PPC was significantly higher in patients with bronchiectasis compared with that in patients without bronchiectasis (aOR, 3.24; 95% CI, 1.57–6.68).
In this study, we demonstrated that the presence of bronchiectasis and its severity significantly increase the risk of PPC after extrapulmonary surgery in patients with airflow limitations. The impact of bronchiectasis on PPC was especially significant in patients who did not use bronchodilators.
Our findings were in line with the results of previous studies, which reported that the presence of bronchiectasis in chronic obstructive pulmonary disease patients is related to increased risk of post-operative exacerbation.
2 Our results added to the evidence of poor outcomes in patients showing coexisting bronchiectasis and airflow limitation with increased PPC after extra-thoracic surgery. Furthermore, our results indicate that patients with bronchiectasis of high severity were 2.74-fold more likely to have PPC. Thus, our study suggests that clinicians should review the presence and extent of bronchiectasis carefully before extra-thoracic surgery.
Bronchial dilation in bronchiectasis leads to impaired mucociliary clearance and failure to adequately clear mucus.
8 Although the exact mechanism via which bronchiectasis increases PPC in the patients with airflow limitation is not well known, altered mucociliary clearance and mucus trapping probably cause atelectasis in bronchiectasis patients by hindering sputum expectoration. Moreover, these phenomena are significantly associated with an increased incidence of post-operative exacerbations. Notably, we found that an increased risk of PPC was attenuated by bronchodilators, as shown in our previous study, where perioperative use of bronchodilators reduced the development of PPC in patients with airflow limitations.
4 The enhanced mucociliary clearance and effective bronchodilation with the use of bronchodilators may have restored decreased mucociliary clearance and assisted in clearing mucus in this population.
8 However, the rate of pneumonia and respiratory failure was similar between patients without bronchiectasis and those with bronchiectasis. Further analysis showed that patients with severe bronchiectasis had a higher rate of pneumonia and respiratory failure than those without bronchiectasis, but this finding did not reach statistical significance. This could be due to insufficient power to detect a significant difference for pneumonia and respiratory failure. Thus, well designed prospective studies with a larger sample size based on our results are needed.
This study had some limitations. First, it was retrospectively performed at a single center, which might limit the generalization of our findings to other institutions. Second, there might be selection bias because patients who performed CT were chosen from our original cohort. Third, sputum culture was performed in one-fifth of bronchiectasis patients, which might have underestimated BSI in some patients.
In conclusion, bronchiectasis was associated with an increased risk of PPC after extra-pulmonary surgery in patients with airflow limitation. Bronchodilators may reduce PPC in bronchiectasis patients with airflow limitation.
Ethics statement
The Institutional Review Board (IRB) of Samsung Medical Center approved this study and permitted the review and publication of patient records (IRB No. 2019-05-045). All data were anonymized before analysis and the need for written informed consent was waived by the IRB due to the retrospective nature of this study.