Abstract
Hospitals need to find a safe and rapid method for respiratory specimen collection as the number of patients suspicious for coronavirus disease -2019 (COVID-19) rapidly grows. Applied with significant infection control and prevention measures, a respiratory specimen collection booth was newly designed. The new respiratory specimen collection booth not only increased COVID-19 testing cases but also decreased personal protective equipment consumption.
Graphical Abstract
Back in December 2019, the outbreak of coronavirus disease-2019 (COVID-19) that first emerged in Wuhan, Hubei Province, China, spread rapidly worldwide and World Health Organization (WHO) declared a pandemic of COVID-19 in March, 2020.1 COVID-19 is an acute respiratory syndrome caused by severe acute respiratory syndrome coronavirus-2 and known to transmit from human to human through droplets, contacts and aerosol. While most confirmed cases of COVID-19 have had a history of close contact with symptomatic carriers diagnosed COVID-19, transmission from an asymptomatic carrier appears to be possible, suggesting the early detection and management of potential cases are crucial to prevent the spread of the virus.2 As the number of patients suspicious for COVID-19 was markedly growing, hospitals needed to increase COVID-19 testing cases. In accordance to the increase in demands, we invented a respiratory specimen collection booth for COVID-19 test and would like to share the experience of using the respiratory specimen collection booth applied with important infection prevention and control measures.
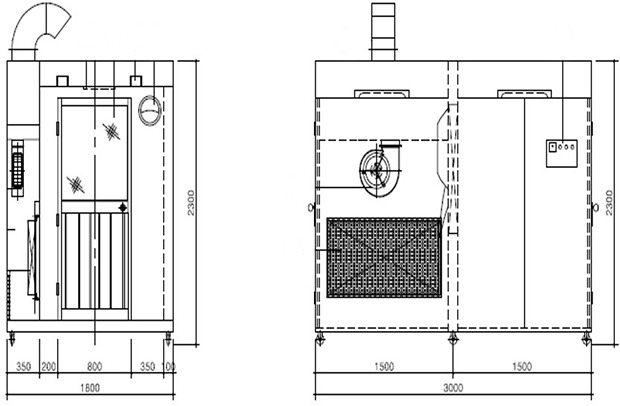
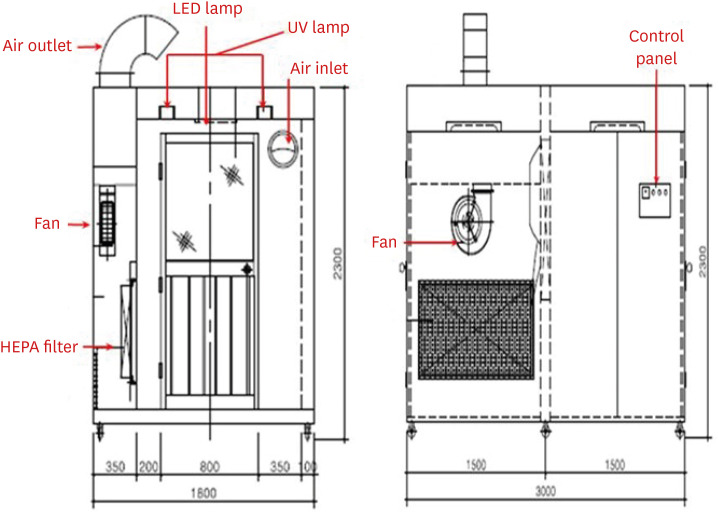
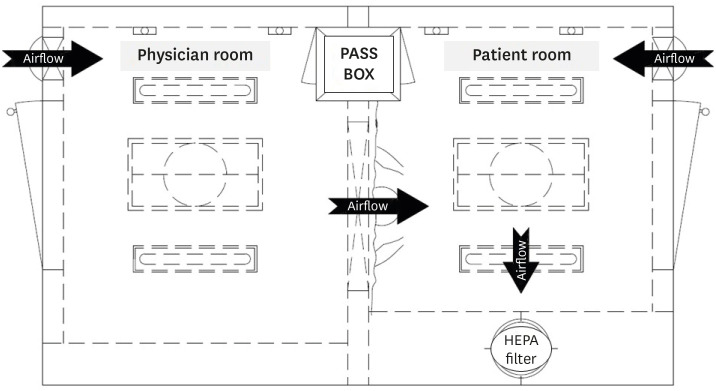
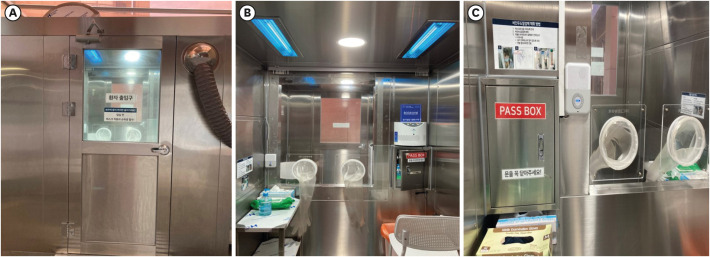
The respiratory specimen collection booth (hereinafter, referred to as ‘the booth’) is made of stainless steel and its size is 2,300 mm in height, 3,000 mm in width, and 1,800 mm in depth (Fig. 1). The booth is divided into two rooms, each for a patient and a physician, with different entrances. There is a transparent acrylic screen on the wall between the rooms and long sleeves with rubber gloves are attached to lower part of the screen. For the environmental control, a negative air pressure machine and a high efficiency particulate air (HEPA) filter are installed in the patient room. The negative air pressure machine, which generates a maximum air volume of 900 m3 per hour, creates negative pressure differential of ≥ 2.5 Pa (Fig. 2). Since the patient room (Fig. 3A) is 5.67 m3 and the air constantly changes 160 times per hour,3 less than 3 minutes are required to remove 99% and 99.9% of the particles from the air. For disinfecting surfaces, two ultraviolet-C (UV-C) lamps (Fig. 3B), each providing 40 uW/cm2, are installed in the ceiling in each room.4 Additionally, there is a pass box (Fig. 3C) between the rooms. The pass box has two doors that could block the airflow when they are closed. There is also a small table which is placed in each room to prepare hand sanitizers, disinfectant wipes and others for packing the specimen container. For the disinfectant wipes, Clinell Universal wipes, containing 0.45% benzalkonium chloride, 0.40% didecyl dimethyl ammonium chloride, and 0.10% polyhexamethylene biguanide, is used.
Yonsei University Severance Hospital is a 2,455-bed tertiary hospital in Seoul, Korea with over 10,000 outpatients and 300 admissions per day on average. There are three screening centers for COVID-19 in the hospital, respectively for adults (outpatient), children (age under 18), and severe patients (emergency room), and two specimen collection booths are installed; one for the adults and the other one for the severe patients. At the screening center, the patient is given a specimen container with a swab and goes into the booth. The physician on the other room collects the sample and the patient puts the specimen container in the pass box. The patient then disinfects high touch surfaces of the room with the disinfectant wipes, while the physician wipes the other surfaces such as rubber gloves, sleeves, and the lower part of the screen. This disinfecting process takes about 1–2 minutes and could lower a risk of viral leakage to outside. After the patient leaves the booth, the physician turns on the UV-C lamps in the patient room, which will be automatically turned off 3 minutes later. Meanwhile, the physician packs the specimen container, which follows Korea Centers for Disease Control and Prevention Guidelines.5 For the next patient, the physician needs to change only the gloves instead of LEVEL D PPE. Around 1 to 2 minutes later when the UV-C lamps are turned off, the next patient is allowed to go inside the booth to make sure patient's safety.
Using the booth, the number of COVID-19 testing increased from 20 to 70 cases per day on average in March 2020, because the total specimen collection time including the environmental cleaning between patients decreased from 20 minutes to at least 5 minutes. It especially shortened the time required in changing personal protective equipment (PPE). Without using the booth, at least two healthcare workers, one physician and one assistant, were required and they needed to change the whole LEVEL D PPE for each patient. However, the booth requires one physician and allows the physician to wear just a respirator, a gown or coverall and disposable gloves, and only the gloves are needed to change for each patient. In consequence, the booth decreased PPE consumption and reduced the cost for PPE. Usually, the price of LEVEL D PPE is around 17 USD per set whereas the PPE required for the booth only costs around 3 USD. Additionally, compared to Drive-thru or Walk-thru Screening Center,6 the booth is not limited to space and weather. This means that it can be installed in any place needed in the hospital. Finally, the environmental cleaning devices including the UV-C lamps, negative air pressure machine and HEPA filter could prevent the cross-infection between patients, and the physician and the patient.
Although all equipment for the environmental cleaning such as the HEPA filter, the UV-C lamps and etc., were used as recommended in the guidelines or the previous research, environmental sampling to evaluate the adequacy of environmental cleaning was not performed. However, the staffs who participated in the specimen collection had daily follow-up with their symptoms suspicious for COVID-19. From March to April 2020, five patients examined at the screening center were confirmed COVID-19 but any of the staffs were not confirmed positive.
Another important consideration is that there is a risk of viral leakage to outside because an anteroom is not available inside the patient room of the booth. However, through the disinfecting process, the patient does not leave the booth immediately after the specimen collection, and because the booth is remote from the waiting area for other patients and the workplace for the staffs, the cross-infection between patients, and the staffs and patients could be prevented.
To strengthen preparedness and response to the COVID-19 outbreak, considerable expenses on medical supplies, devices and human resources for screening and treating patients diagnosed COVID-19 should be primarily supported.7 Financially, high initial cost of installation of the booth could be a burden for many hospitals. However, the initial cost is almost the same as consuming around 100 LEVEL D PPE per day for two weeks and the booth could rather save the budget for the COVID-19 response in the long term. Although the booth still needs a few improvements in the disinfecting measures, it has played a significant role in efficiently conducting the sample collection for COVID-19 test. We believe that sharing the experience of operating the booth could contribute to the global society in response to the massive outbreak of any type of new merging infectious disease in the future as well as COVID-19.
ACKNOWLEDGMENTS
We would like to dedicate this article to the members of the Department of Infection Control and the Department of Laboratory Medicine, Severance Hospital, who devoted considerable effort to design and install the respiratory specimen collection booth while coping with the current massive outbreak of COVID-19. This article could not have been completed without their invaluable aid.
References
1. World Health Organization. Coronavirus Disease 2019(COVID-19) Situation Report-51. Geneva: World Health Organization;2020.
2. Gao Z, Xu Y, Sun C, Wang X, Guo Y, Qiu S, et al. A systematic review of asymptomatic infections with COVID-19. J Microbiol Immunol Infect.

3. Centers for Disease Control and Prevention. Guidelines for environmental infection control in health-care facilities (2003). Updated 2019. Accessed September 7, 2020. https://www.cdc.gov/infectioncontrol/guidelines/environmental/appendix/air.html.
4. Cadnum JL, Li DF, Jones LD, Redmond SN, Pearlmutter B, Wilson BM, et al. Evaluation of ultraviolet-c light for rapid decontamination of airport security bins in the era of SARS-CoV-2. Pathog Immun. 2020; 5(1):133–142. PMID: 32582873.

5. Hong KH, Lee SW, Kim TS, Huh HJ, Lee J, Kim SY, et al. Guidelines for laboratory diagnosis of coronavirus disease 2019 (COVID-19) in Korea. Ann Lab Med. 2020; 40(5):351–360. PMID: 32237288.

6. Kwon KT, Ko JH, Shin H, Sung M, Kim JY. Drive-through screening center for COVID-19: a safe and efficient screening system against massive community outbreak. J Korean Med Sci. 2020; 35(11):e123. PMID: 32193904.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download