Healthcare workers (HCWs) are susceptible to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection from infected patients. Therefore, ensuring adequate personal protective equipment (PPE) for HCWs is important. Experts recommend HCWs to use PPE including N95-equivalent respirators, gowns, gloves, and eye protection while providing care for patients with suspected or confirmed coronavirus disease 2019 (COVID-19), although respirator use during routine care is controversial.
12 Universal PPE use including N95 respirator, face shield/goggles, gown, and gloves for HCWs is impossible given the shortage of PPE and fatigue of HCWs in routine outpatient work. Despite the screening and triaging strategy, HCWs are at risk of exposure to asymptomatic or pauci-symptomatic patients with COVID-19, without adequate PPE. Transmission risk in outpatient clinic settings may vary from that in the general ward, emergency room, or intensive care unit given the differences in duration of contact, intimacy of contact, adequate PPE use, and performing aerosol-generating procedures. However, the risk of COVID-19 transmission from infected outpatients to HCWs remains poorly understood. Therefore, we evaluated the risk of infection of HCWs from patients with confirmed COVID-19 in the outpatient clinic setting.
This study was performed in a 2,700-bed tertiary care hospital in Seoul, Korea, that employs 8,800 HCWs and has 12,000 outpatient visits per day. We recommend all HCWs to wear filtering face piece (FFP) 2-equivalent masks, gloves, goggles or face shields, and gowns regardless of patient symptoms in high-risk departments such as emergency rooms, endoscopy rooms, the otolaryngology clinic, and the dental clinic. FFP2 masks had a filter efficacy of 94% for 0.3 μm particles meeting the European Standard EN 149.
Information on whether a patient with confirmed COVID-19 visited our hospital during the infectious period was obtained from Korea Centers for Disease Control and Prevention (KCDC). From January to September 2020, we had conducted contact-tracing for confirmed COVID-19 patients who visited our outpatient clinics. We identified all contacts by reviewing the closed-circuit television footage and interviewing the index patient and contacts. Close contact was defined as 1) being within approximately 6 feet of a patient with confirmed COVID-19 for at least 15 minutes or 2) direct contact without the use of appropriate PPE (N95 or FFP2 equivalent respirator, face shield/goggles, gown, and gloves). All close contacts were placed under quarantine for 14 days. HCWs and inpatients or guardians who came into contact underwent SARS-CoV-2 polymerase chain reaction (PCR) testing with daily symptom monitoring. The Public Health Center educated the discharged patients or guardians coming into contact and performed PCR testing or placed them under quarantine, as necessary.
A total of 8 outpatients with a confirmed COVID-19 diagnosis visited our outpatient clinic during the infectious period (
Table 1 and
Fig. 1). The median time spent in the hospital and the median examination time for the confirmed patients was 164 minutes (interquartile range [IQR], 92-179 min) and 6 minutes (IQR, 3-10 min), respectively. The KCDC notified us that the patient 2 tested positive on the SARS-CoV-2 PCR test when he was waiting for meeting the doctor after taking EKG, X-ray, and blood sampling. At the time of visiting our hospital, 5 patients (62.5%) were asymptomatic, and 3 patients (37.5%) were pauci-symptomatic. Except 3 patients (patients 2, 5, and 7), other 5 patients wore masks in the hospital. The patient 2 was unmasked for 16 minutes for eating breakfast. The patient 5 was unmasked for < 1 minute while consuming a drink, 5 minutes while talking on the phone, and 10 minutes while receiving dental treatment. The patient 5 received supportive periodontal treatment in the dental clinic which could generate aerosols, and 2 HCWs who were in the treatment room wore FFP2-equivanlent masks, gowns, and gloves but not goggles. The patient 7 visited our hospital 2 times, once on the 24th and then on the 26th of August, 2020. On the first visit, he was unmasked for 27 minutes while eating lunch. On the second visit, he was unmasked for 11 minutes while undergoing esophagogastroduodenoscopy; the physician performing the procedure was wearing an FFP2-equivalent mask, gown, and gloves but not goggles, and the nurse was not wearing gloves.
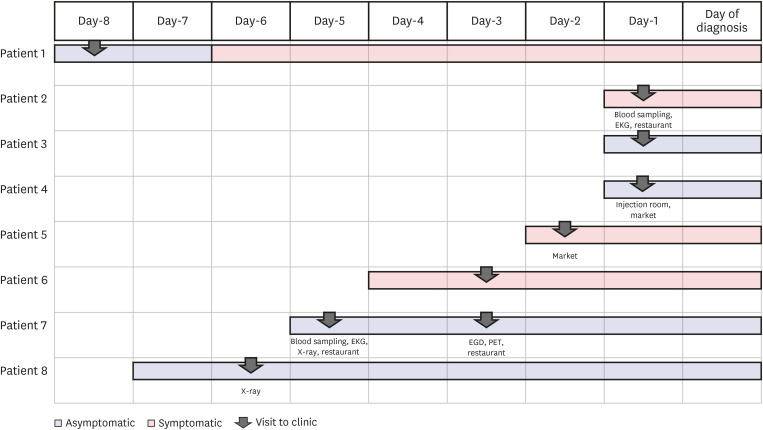 | Fig. 1
Chronology of symptom onset, clinic visit, and coronavirus disease 2019 diagnosis.
EGD = esophagogastroduodenoscopy, EKG = electrocardiogram, PET = positron emission tomography.

|
Table 1
Characteristics of patients with confirmed COVID-19 and the number of HCWs who they had contact with
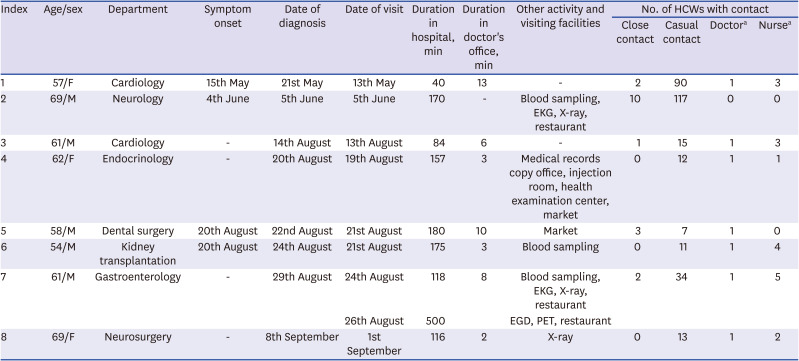
|
Index |
Age/sex |
Department |
Symptom onset |
Date of diagnosis |
Date of visit |
Duration in hospital, min |
Duration in doctor's office, min |
Other activity and visiting facilities |
No. of HCWs with contact |
|
Close contact |
Casual contact |
Doctora
|
Nursea
|
|
1 |
57/F |
Cardiology |
15th May |
21st May |
13th May |
40 |
13 |
- |
2 |
90 |
1 |
3 |
|
2 |
69/M |
Neurology |
4th June |
5th June |
5th June |
170 |
- |
Blood sampling, EKG, X-ray, restaurant |
10 |
117 |
0 |
0 |
|
3 |
61/M |
Cardiology |
- |
14th August |
13th August |
84 |
6 |
- |
1 |
15 |
1 |
3 |
|
4 |
62/F |
Endocrinology |
- |
20th August |
19th August |
157 |
3 |
Medical records copy office, injection room, health examination center, market |
0 |
12 |
1 |
1 |
|
5 |
58/M |
Dental surgery |
20th August |
22nd August |
21st August |
180 |
10 |
Market |
3 |
7 |
1 |
0 |
|
6 |
54/M |
Kidney transplantation |
20th August |
24th August |
21st August |
175 |
3 |
Blood sampling |
0 |
11 |
1 |
4 |
|
7 |
61/M |
Gastroenterology |
- |
29th August |
24th August |
118 |
8 |
Blood sampling, EKG, X-ray, restaurant |
2 |
34 |
1 |
5 |
|
26th August |
500 |
EGD, PET, restaurant |
|
8 |
69/F |
Neurosurgery |
- |
8th September |
1st September |
116 |
2 |
X-ray |
0 |
13 |
1 |
2 |

On thorough contact-tracing, we identified 453 close and casual contacts (317 HCWs and 136 patients or guardians). Among 317 HCWs that came in contact, 18 HCWs (16 HCWs with direct contact without adequate PPE and 2 HCWs with prolonged contact) were in close contact. HCWs with close contact were placed under self-isolation for 2 weeks and subsequently underwent a PCR test on the 14th day before returning to work. Remaining 299 HCWs had nasopharyngeal swab tests scheduled 3 days from exposure or every 3 days depending on the exposure risk. Per the test results, none of the 317 HCWs who came in contact had a confirmed COVID-19 diagnosis (0.00%; 95% confidence interval, 0.00%–0.01%). Of 136 patients or guardians with contacts, 13 hospitalized patients underwent SARS-CoV-2 PCR testing and all were negative.
During the study period, 3 of 8,800 HCWs were confirmed COVID-19 in our hospital. According to the interview and the closed-circuit television footage, none had contact with confirmed patients in the hospital. One HCW who was wife of the patient 5 had only household exposure with him.
COVID-19 infections in HCWs are frequently presumed to be acquired by contact with COVID-19 patients. The US Centers for Disease Control and Prevention reported that 55% of infected HCWs had contact with COVID-19 patients only in health care settings.
3 However, exposure in household or community settings also contributes to infections among HCWs.
345 A study from Belgium that used SARS-CoV-2 antibody screening reported that rate of positive infections was higher among HCWs with household contacts than those without household contacts.
5 The transmission risk among household members could be as high as 40%.
678 In our study, there were no secondary infections among HCWs with contact in healthcare settings. Therefore, the safety of HCWs not only at work but also in the community is important.
The risk of COVID-19 transmission in outpatient clinics may differ from those of other units. The proportion of HCWs working in outpatient clinics among all infected HCWs is reported to be as low as 2.7%–6.9%.
49 The brief interaction time, low rate of performing aerosol-generating procedures, and low frequency of direct contact in outpatient clinics could be possible reasons for the low transmission rate of COVID-19 in such settings. Previous studies have reported that using N95-equivalent masks and face shields were associated with lower transmission rate.
10 However, in our study, most HCWs wore only surgical masks at the time of exposure and even during aerosol-generating procedures; they did not wear goggles or face shields or gloves. Our findings support the low risk of infection among HCWs with the use of standard precautions such as wearing surgical masks and maintaining hand hygiene. Nevertheless, when providing care for patients with suspected or confirmed COVID-19, especially during aerosol-generating procedures, using adequate PPE including N95-equivalent masks and face shields is essential.
Our study has several limitations. First, the retrospective, single-centre design implies limited generalizability of results, and this is the study with a limited sample size from low prevalence country. Second, not all patients or guardians who were already discharged and later identified to have contact exposure had undergone PCR testing for SARS-CoV-2. The Public Health Center educated them about monitoring their symptoms, and the SARS-CoV-2 PCR test was not performed unless these patients presented any symptoms. The transmission rate of COVID-19 in these groups could be underestimated in our study. Finally, Ct values for SARS-CoV-2 genes of confirmed COVID-19 patients were limited because we were informed of the outside SARS-CoV-2 PCR test results. When visiting our hospital, the confirmed COVID-19 patient might not have been contagious.
In conclusion, the risk of transmission from patients with confirmed COVID-19 to HCWs in outpatient clinics is very low. In addition, using standard precautions such as wearing a surgical mask and maintaining good hand hygiene protect HCWs from infection in outpatient clinic settings.






 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download