INTRODUCTION
The coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) had spread throughout the world, and the total cases numbered more than 17 million and 680,000 deaths by COVID-19 as of August 3, 2020.
1 On January 20, 2020, a Chinese traveler from Wuhan, China, was identified as the first COVID-19 case in Korea, after which only 30 cases of COVID-19 were reported until February 20 and were based on visitors to the country and contact with them. After an outbreak associated with a religious group in Daegu Metropolitan city, the number of new patients per day rapidly increased to a maximum of 813 from late February to late March. The government of Republic of Korea had increased the response level for public health emergency to level 3 (from level 0 to level 3) on February 23, and had implemented high-intensity social distancing until May 5.
2 Furthermore, the government applied the K-quarantine model ‘3T policy (Test-Trace-Treat)’ system that included a rapid and exact test for COVID-19, investigation of the epidemic using information and communication technology (ICT), and an isolation and care program according to severity.
3
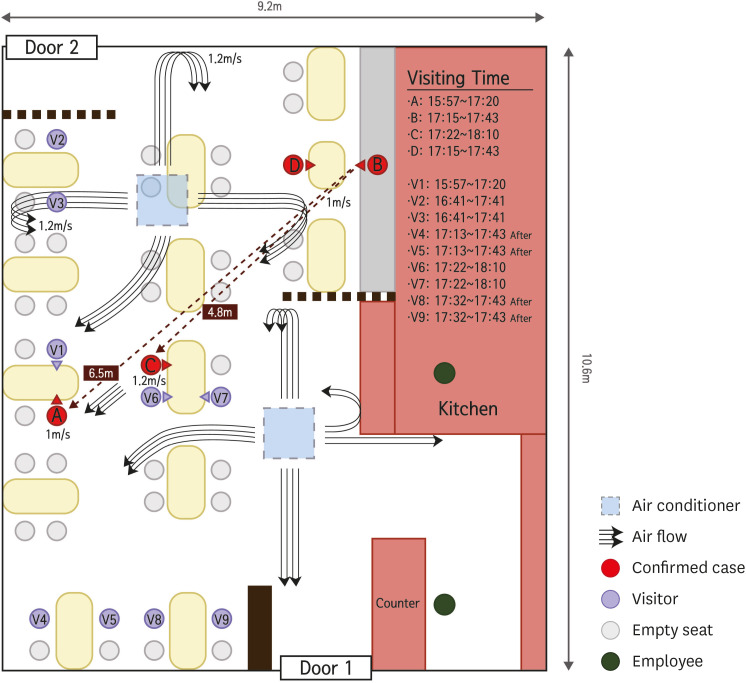
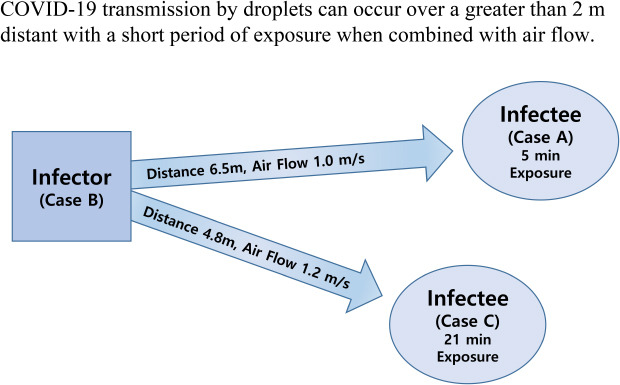
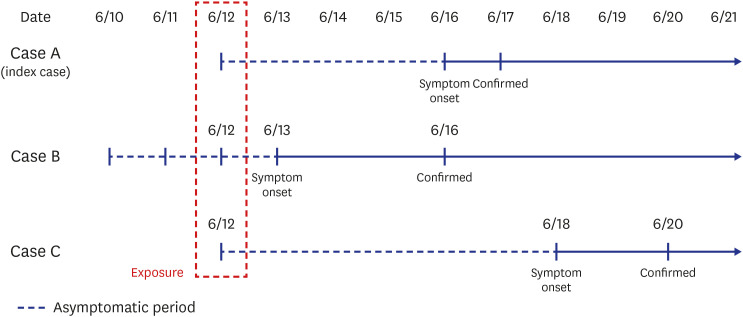
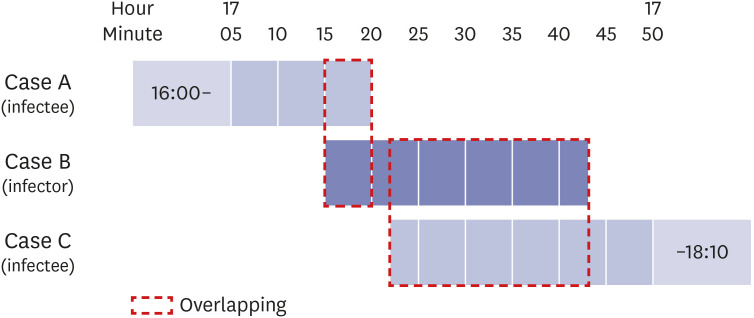
On June 17, there was a new confirmed COVID-19 case (index case, case A) in Jeonju, Korea, considered as transmitted by droplets at 6.5 m away from the infector and 5 minutes exposure in a restaurant with air conditioning. It is important to know how SARS-CoV-2 is transmitted between people in various situations. We share these investigation results as a reference to update guidelines involving prevention, tracing, and quarantine for control of this pandemic infectious disease.
DISCUSSION
The SARS-CoV-2 virus is mainly transmitted through respiratory droplets emitted from an infector's coughing, sneezing, talking, and normal breathing and upon close contact between people.
15 These droplets are typically divided into large and small sizes (also called aerosol) based on a diameter of 5 μm. The large particles (also called droplets) tend to settle within 1–2 m of their origin due to gravitational force, and the settling velocity is proportional to the particle diameter.
16 Therefore, social distancing requires a minimum of 1–2 m to avoid contact with a virus-containing respiratory droplet. In the situation of no effective treatment drug or vaccine, the most important personal methods to prevent or control this pandemic of COVID-19 are social distancing, use of a mask (if social distancing cannot be maintained), and handwashing.
Recently, it has been suggested that COVID-19 can be spread through not only droplets or contact, but also airborne transmission. An experimental study showed that the COVID-19 virus in aerosol particles remained viable during 3 hours and 16 hours.
17 Morawska and Milton, together with their co-authors, 239 scientists, strongly suggested the possibility of airborne transmission of COVID-19 based on several preprint findings, though there has been no peer review of this research.
18 The last updated version of the WHO scientific brief reported on July 9, 2020 reported that airborne transmission by aerosols is rare, and SARS-CoV-2 is spread primary between people through droplets or close contact. However, the possibility of aerosol transmission in crowded indoor spaces has been suggested in combination with droplet transmission.
19
In this outbreak, the distances between infector and infected persons were 4.8 and 6.5 m, both farther than the generally accepted 2 m droplet transmission range. This is some of the first evidence of airborne transmission. At the field investigation, we assumed the possibility of long-distance droplet movement by air flow. Dbouk and Drikakis reported results of computational fluid dynamics showing that most droplets settle within 1–2 m in the absence of airflow. However, with a 4 km/hr or 15 km/hr wind, droplets could travel 6 m after 5 or 1.6 seconds, respectively.
20 In the presented case, the air flow from infector to infectee showed a 1.0–1.2 m/sec (3.6–4.3 km/hr) velocity, indicating the need for 6.5 seconds of contact to transmit droplets from the infector to the index case. Only the visitors (cases A and C) sitting in the air flow path of case B were infected with COVID-19, while other visitors (V2, V3) closer to the infector for a longer period of time but in the absence of direct air flow did not become infected. In addition, the visitors sitting at tables with cases A and C (V1, V6, and V7) were not infected with COVID-19 because they faced away from the infector’s face. These findings strongly suggest that this outbreak occurred by droplet transmission exceeding a 2 m distance and excluded contact and fomite transmission. This transmission pattern is similar with the outbreak of a restaurant with air conditioning in Guangzhou, China.
21 In this article, the authors concluded that the most likely transmission was done by droplet and also emphasized the direction of air flow.
Without the K-quarantine model (test-trace-treat) and EISS, it may have been very difficult to establish an infection chain of this outbreak because the incubation period of COVID-19 has a wide range from 1 to 14 days, and the exposure occurred only once for 5 minutes at a 6.5 m distance. Based on this system, the infector was identified within 2 days after the index case was confirmed. This short period of identification could simplify identification of close contacts and reduce outbreak size by quarantining of all close contacts. In most COVID-19 outbreak situations, identification of the infection chain is difficult or almost impossible. An outbreak for which an infectious source cannot be determined may be due to failure of consideration of a short period of exposure or of clearly verifying the movements of the confirmed person. In addition, it suggests that some suspected airborne transmission reports may be misinterpreted based on lack of awareness of the long transmission mechanism of droplets.
The guideline for control of droplet transmission over a long distance (above 2 m) by air flow in indoor settings are similar to those of airborne transmission and comprise sufficient ventilation and social distancing (avoid overcrowding, maintain distance between people).
22 However, if there is high possibility of transmission by aerosol or droplet transmission over a long distance, N95 respiratory or equivalent masks are needed not only in health care settings, but in any indoor environment. In Korea, the Ministry of Food and Drug Safety is concerned with approval of medical mask like KF99, KF94, and KF80 and developed a type of mask named KF-AD (anti-droplet) for COVID-19. Any such mask, including KF and surgical, that can protect against droplets should be sufficient for preventing droplet transmission.
According to this case, additional considerations need to occur for COVID-19 prevention and control. The first is that transmission in an indoor setting is possible at a distance greater than 2 m with a short period of exposure (five minutes), and selection of close contacts in contact tracing should be changed. When epidemiological field investigation of an indoor environment is needed, it is necessary to assess the seating arrangement and operation and location of fans (including ceiling fans) or air conditioners with wind direction and velocity. It is also necessary to ventilate frequently for management of indoor air or to apply a ventilation system or forced ventilation method if natural ventilation is not possible. Furthermore, the distance between tables at an indoor restaurant or cafeteria should be greater than 1–2 m, or installation of a wind partition should be considered based on air flow. In addition, in indoor settings such as restaurants, masks should be removed only during meals and should be worn before and after eating, while conversation during meals and loud talking or shouting should be avoided. In the long term, installation of separate rooms or bulkheads for indoor settings should be considered to prevent transmission of airborne and droplet infectious diseases.
There are some limitations to this study. First, we did not assess air flow using computational fluid dynamics. In addition, the air flow measurement can't reflect all the same situations because opening of doors and motions of cases and visitors were not reproduced. However, air flow direction and velocity were identified between the infector and infectees using an anemometer in the most similar environment as possible. Second, environmental samples were collected at 11 days after the inspector visit. Though all these results were negative, this was not proof against airborne transmission.
In conclusion, droplet transmission can occur at a distance greater than 2 m if there is direct air flow from an infected person in an indoor setting. Therefore, updated guidelines for quarantine and environmental management of COVID-19 are needed until approval of an effective treatment drug or vaccine.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download