Clostridioides difficile infection (CDI) is one of the most common hospital-acquired infections, with symptoms ranging from mild diarrhea to life-threatening pseudomembranous colitis.
1 In the US, approximately 223,900 CDI cases occur and more than 12,500 patients with CDI die annually.
2 Antibiotics have a significant and long-lasting effect on the intestinal microbiota and reduce colonization resistance against pathogens, including
C. difficile.
3 One of the most important predisposing factors for CDI is exposure to high-risk antimicrobial agents including fluoroquinolone (FQ), extended-spectrum cephalosporins (ESC), and clindamycin.
14 In addition, the use of any antibiotic has been associated with CDI development, and even short-term antibiotic use may increase the risk of CDI.
56 Patients are prone to developing CDI three months after cessation of antibiotic therapy, and the risk is the highest during the first month after stopping antibiotic use.
7
Domestic data on high-risk antibiotics for treating CDI in Korean hospitals are limited.
8 Because any antibiotic can predispose an individual to CDI, it is important to identify high-risk antimicrobial agents in Korean hospitals to develop a proper strategy to reduce CDI.
4 To this end, we conducted the present study to define the association between antibiotic consumption and CDI incidence at the hospital level.
A single-center retrospective study was conducted at a tertiary-care hospital with 859 beds in Korea. Positive results for C. difficile toxin genes (tcdA or tcd B, cdtA, or cdt B) using multiplex PCR, positive results for C. difficile toxin A & B assay (VIDAS C. difficile Toxin A & B; Biomerieux SA, Marcy I’Etoile, France), antibiotic prescriptions, patient days, and number of admissions were collected from inpatients between 2009 and 2013. Antimicrobial stewardship programs remained largely unchanged during the study period. The most prominent strategy for antimicrobial stewardship programs in the study hospital was restrictive measures for designated antimicrobials.
We defined CDI when a patient showed positive results for
C. difficile toxin genes and/or
C. difficile toxin A & B assay while having unformed stools more than three times a day on consecutive days.
5 We included multiple CDI episodes from one patient, but only one CDI episode was included per hospitalization. In addition, among patients with CDI, we included only hospital-acquired CDI (HA-CDI) for the analysis. Patients who developed diarrhea at least 72 hours after hospitalization or within 2 months from the last discharge and did not reside in a long-term care facility were diagnosed with HA-CDI.
9 CDI incidence was defined as the number of cases per 10,000 admissions.
Prescription records for antibiotic classes such as clindamycin, FQ, beta-lactam/beta-lactamase inhibitor (BL/BLI), 1st generation cephalosporin (1st CEP), 2nd generation cephalosporin (2nd CEP), ESC, penicillin, macrolide, trimethoprim/sulfamethoxazole (SXT), glycopeptide, rifampicin, aminoglycoside, and carbapenem were collected. In addition, we collected data for levofloxacin, moxifloxacin, and piperacillin/tazobactam (TZP) for a detailed evaluation of the effect of FQ and BL/BLI. Only systemic agents with oral or parenteral administration routes were included, while topical agents were excluded. ESC comprised 3rd and 4th generation cephalosporins. The amount of antibiotic consumption was calculated by defined daily dose (DDD) using the World Health Organization's Anatomical Therapeutic Chemical classification,
10 and this was then standardized for 1,000 patient-days.
We classified clindamycin, FQ, BL/BLI, 1st CEP, 2nd CEP, ESC, penicillin, macrolide, and SXT as antibiotics frequently associated with CDI and others as antibiotics rarely associated with CDI.
4 Spearman's rank correlation analysis was used to describe the relationship between antibiotic consumption and HA-CDI incidence. Matched-month and one-month delay approaches were applied to evaluate both the immediate and delayed effects of antibiotics on CDI occurrence, respectively. Statistical significance was defined as
P < 0.05. All analyses were performed using SPSS 24.0 (IBM Corporation, Armonk, NY, USA).
HA-CDI was diagnosed at 45.33-58.10 cases per 10,000 admissions at the hospital during the study period.
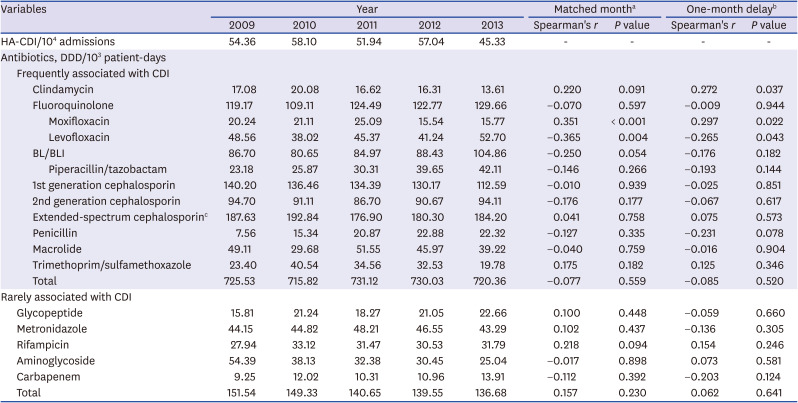
Table 1 shows the correlation between HA-CDI incidence and antibiotic consumption. When analysis was performed using the matched-month approach, moxifloxacin consumption (
r = 0.351,
P < 0.001) displayed a significant positive correlation with HA-CDI incidence. Contrarily, levofloxacin consumption (
r = −0.365,
P = 0.004) was negatively correlated with HA-CDI incidence; BL/BLI consumption (
r = −0.250,
P = 0.054) displayed a negative correlation with HA-CDI incidence with marginal significance. Other antibiotics known to be frequently associated with CDI, such as clindamycin, ESC, penicillin, macrolide, and SXT, did not have any effect on HA-CDI incidence. None of the antibiotics known to be rarely associated with CDI showed a significant correlation with HA-CDI incidence.
Table 1
Correlation between hospital-acquired Clostridioides difficile infection incidence and antibiotic consumption
|
Variables |
Year |
Matched montha
|
One-month delayb
|
|
2009 |
2010 |
2011 |
2012 |
2013 |
Spearman's r
|
P value |
Spearman's r
|
P value |
|
HA-CDI/104 admissions |
54.36 |
58.10 |
51.94 |
57.04 |
45.33 |
- |
- |
- |
- |
|
Antibiotics, DDD/103 patient-days |
|
|
|
|
|
|
|
|
|
|
Frequently associated with CDI |
|
|
|
|
|
|
|
|
|
|
|
Clindamycin |
17.08 |
20.08 |
16.62 |
16.31 |
13.61 |
0.220 |
0.091 |
0.272 |
0.037 |
|
|
Fluoroquinolone |
119.17 |
109.11 |
124.49 |
122.77 |
129.66 |
−0.070 |
0.597 |
−0.009 |
0.944 |
|
|
|
Moxifloxacin |
20.24 |
21.11 |
25.09 |
15.54 |
15.77 |
0.351 |
< 0.001 |
0.297 |
0.022 |
|
|
|
Levofloxacin |
48.56 |
38.02 |
45.37 |
41.24 |
52.70 |
−0.365 |
0.004 |
−0.265 |
0.043 |
|
|
BL/BLI |
86.70 |
80.65 |
84.97 |
88.43 |
104.86 |
−0.250 |
0.054 |
−0.176 |
0.182 |
|
|
|
Piperacillin/tazobactam |
23.18 |
25.87 |
30.31 |
39.65 |
42.11 |
−0.146 |
0.266 |
−0.193 |
0.144 |
|
|
1st generation cephalosporin |
140.20 |
136.46 |
134.39 |
130.17 |
112.59 |
−0.010 |
0.939 |
−0.025 |
0.851 |
|
|
2nd generation cephalosporin |
94.70 |
91.11 |
86.70 |
90.67 |
94.11 |
−0.176 |
0.177 |
−0.067 |
0.617 |
|
|
Extended-spectrum cephalosporinc
|
187.63 |
192.84 |
176.90 |
180.30 |
184.20 |
0.041 |
0.758 |
0.075 |
0.573 |
|
|
Penicillin |
7.56 |
15.34 |
20.87 |
22.88 |
22.32 |
−0.127 |
0.335 |
−0.231 |
0.078 |
|
|
Macrolide |
49.11 |
29.68 |
51.55 |
45.97 |
39.22 |
−0.040 |
0.759 |
−0.016 |
0.904 |
|
|
Trimethoprim/sulfamethoxazole |
23.40 |
40.54 |
34.56 |
32.53 |
19.78 |
0.175 |
0.182 |
0.125 |
0.346 |
|
|
Total |
725.53 |
715.82 |
731.12 |
730.03 |
720.36 |
−0.077 |
0.559 |
−0.085 |
0.520 |
|
Rarely associated with CDI |
|
|
|
|
|
|
|
|
|
|
Glycopeptide |
15.81 |
21.24 |
18.27 |
21.05 |
22.66 |
0.100 |
0.448 |
−0.059 |
0.660 |
|
Metronidazole |
44.15 |
44.82 |
48.21 |
46.55 |
43.29 |
0.102 |
0.437 |
−0.136 |
0.305 |
|
Rifampicin |
27.94 |
33.12 |
31.47 |
30.53 |
31.79 |
0.218 |
0.094 |
0.154 |
0.246 |
|
Aminoglycoside |
54.39 |
38.13 |
32.38 |
30.45 |
25.04 |
−0.017 |
0.898 |
0.073 |
0.581 |
|
Carbapenem |
9.25 |
12.02 |
10.31 |
10.96 |
13.91 |
−0.112 |
0.392 |
−0.203 |
0.124 |
|
Total |
151.54 |
149.33 |
140.65 |
139.55 |
136.68 |
0.157 |
0.230 |
0.062 |
0.641 |

Using the one-month delay approach, we found that clindamycin (
r = 0.272,
P = 0.037) and moxifloxacin consumption (
r = 0.297,
P = 0.022) displayed significant positive correlation with HA-CDI incidence. Levofloxacin consumption presented a negative correlation with HA-CDI incidence (
r = −0.265,
P = 0.043). None of the other antibiotics presented any significant effect on HA-CDI incidence in the one-month delay analysis (
Fig. 1).
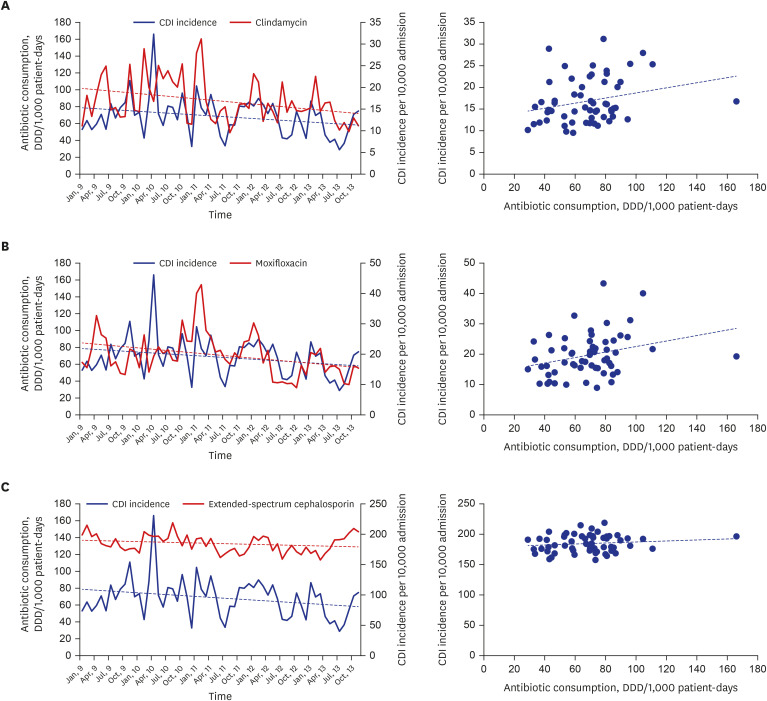 | Fig. 1
Spearman's correlation and consumption-over-time plots by one-month delay analysis, (A) for lincosamide consumption and CDI incidence (Spearman's r = 0.272, P = 0.037), (B) for moxifloxacin consumption and CDI incidence (Spearman's r = 0.297, P = 0.022), and (C) for extended-spectrum cephalosporin consumption and CDI incidence (Spearman's r = 0.075, P = 0.573).
CDI = Clostridioides difficile infection, DDD = defined daily dose.

|
Although previous studies in other countries have reported the association of FQ, ESC, and carbapenem consumption with CDI incidence, we found that clindamycin and moxifloxacin consumption affected CDI incidence in the study hospital.
1112 Possibly, these antibiotics imposed strong selective pressures on
C. difficile, considering the high rates of resistance of large-cluster isolates to clindamycin and moxifloxacin reported in our previous study.
8 The development of
C. difficile resistance appears to play an important role in CDI due to the presence of strains with increased virulence.
1314 A strain of clindamycin-resistant
C. difficile was implicated in a large outbreak in the early 1990s, and FQ-resistant hypervirulent NAP1/BI/O27 was an important factor in outbreaks in the 2000s.
1314 Our study showed that levofloxacin consumption had a negative correlation with CDI incidence. One plausible reason for this finding might be a trade-off phenomenon between levofloxacin and moxifloxacin. Many physicians may consider them as having the same spectrum and effects. Similarly, the negative correlation between BL/BLI consumption and CDI incidence might be attributable to the trade-off effect between other risky antibiotics such as clindamycin and moxifloxacin throughout the hospital. Further studies are necessary to clarify the effect of levofloxacin and BL/BLI on the incidence of CDI.
Several studies have reported that HA-CDI incidence can be successfully controlled by restricting the use of these high-risk antibiotics.
151617 Given that restricting high-risk antimicrobial agents has been more effective in reducing CDI incidence than other measures, such as improvements in hospital infection control, antimicrobial stewardship for high-risk agents has been considered to be a central component of CDI control.
616 Unfortunately, very few Korean hospitals perform monitoring and intervention for high-risk antimicrobials for CDI.
18 Therefore, it is necessary to implement antimicrobial stewardship program interventions targeting high-risk antibiotics for CDI in Korean hospitals.
There are some potential limitations to our study. First, we evaluated the correlation between antibiotic use and CDI incidence at the hospital level; therefore, the results cannot be extrapolated to the patient level. In addition to individual antibiotic usage patterns, other important risk factors for individuals, such as age, underlying comorbidities, and chemotherapeutic agent use, were not analyzed. Second, this study was performed in a single center; thus, the findings cannot be generalized. Third, antibiotic consumption was measured by DDD instead of days of therapy. The antibiotic use of pediatric patients or patients with renal impairment might not be calculated correctly.
19 Finally, because CDI was diagnosed by stool culture or toxin assay only when requested, CDI incidence may have been underestimated.
In conclusion, CDI incidence was positively correlated with clindamycin and moxifloxacin use. It is necessary to implement proper antimicrobial stewardship programs targeting high-risk antibiotics for CDI in Korean hospitals.
Ethics statement
The study protocol was approved by the Institutional Review Boards of the Hanyang University Hospital (2015-01-015), and the requirement for written informed consent from patients was waived due to the retrospective nature of the study and its impracticability.
Go to :







 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download