INTRODUCTION
The World Health Organization (WHO) declared the coronavirus disease 2019 (COVID-19) outbreak a Public Health Emergency of International Concern (PHEIC) on January 30, and a pandemic on March 11, 2020. The PHEIC declaration is the 6th time the WHO has declared a PHEIC since the International Health Regulations (IHR) came into force in 2005.
12 As of June 19, there were 10,193,723 confirmed cases of COVID-19 and the death toll reached 503,867 worldwide.
3
Reports on symptoms of COVID-19 are non-specific and known to vary from asymptomatic or mild to severe or fatal.
4 The symptoms of 1,000 patients hospitalized with COVID-19 in New York were in the order of cough (73.2%), fever (72.8%), and dyspnea (60.1%). Studies conducted in China mainly reported fever, dry cough, myalgia, fatigue, dyspnea, and anorexia, and rare cases of diarrhea.
56 As a result of comparing the clinical symptoms of 36 children with COVID-19 in China to those of adults, there was a difference in the onset of symptoms such as fever (36% for children and 86% for adults), and the proportion of children with no symptoms was higher than that of adults.
7 A study conducted in Daegu, Korea reported that prognostic factors such as body temperature above 37.8°C, diabetes mellitus were independent predictors of severe cases of COVID-19, stressing assessment of the factors and appropriate interventions in high-risk group.
8 Based on these findings, we can infer that clinical symptoms and prognosis vary depending on age and severity of patients who test positive for COVID-19.
The majority of prior studies took place in China, Korea, and the United States, wherein they were limited to analyzing the outbreak or transmission in a hospital or a specific region. The Organization for Economic Cooperation and Development reported that some countries in Central Asia mitigated the effects of COVID-19 through strict lockdown regimes for some populations. As of the first week of May, when COVID-19 cases surged worldwide, mortality per million was 1.9 in Afghanistan, 1.5 in Kyrgyzstan, 1.3 in Kazakhstan, and 0.3 in Uzbekistan, which were far below those in Germany (79.4), France (379.3), Italy (474.8), or Spain (540.4).
9 A study conducted in Kazakhstan analyzed the incidence, mortality, case-fatality rates, and predictive modeling, but lacked information on the clinical symptoms of COVID-19 patients.
10
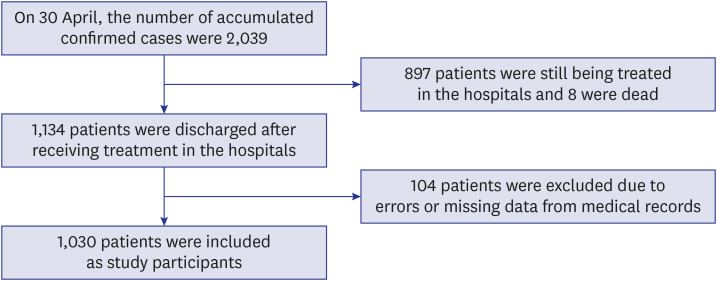
In Uzbekistan, the target country of this study, the first COVID-19 confirmed cases were reported on March 15, 2020. In total, 5,697 confirmed cases and 19 deaths have been reported as of June 19. Uzbekistan quarantined all schools, kindergartens, and universities on March 16. From March 15 to May 31, the number of confirmed cases continued to increase with several peaks (
Fig. 1). Self-isolation in Tashkent, Nukus, and regional centers became mandatory, and it was forbidden to leave home, except in cases of emergency from April 6. Despite the strengthening of quarantine, the highest peak was on April 14. The daily curve of the number of new cases among study participants was affected by the number of those returned from other countries. The number of participants from abroad peaked on March 27, April 5, and April 9, similar to the trend of the total number of new cases in other regions. After the highest peak, we observed the similarity between total cases and domestic infection on April 17 and April 25.
Fig. 1
The curve of the daily number of new cases among study participants by international exposure and national responses in the Republic of Uzbekistan (n = 1,030).
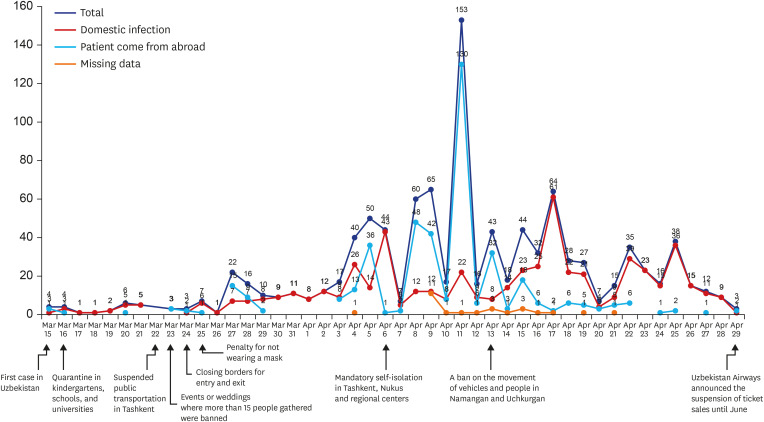
The cumulative number of confirmed cases in Uzbekistan, Norway and Malaysia as of June 30 were similar, however the case fatality rate of COVID-19 in Uzbekistan was 0.3%, lower than that of Norway (2.8%) and Malaysia (1.4%).
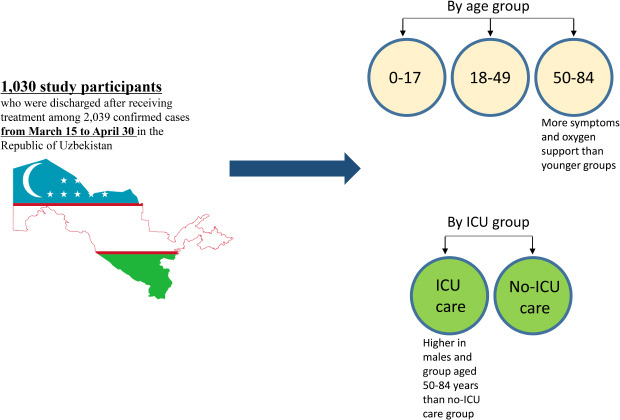
3 Uzbekistan is also one of the leading countries that applied intensive social distancing policies. To prevent the transmission of COVID-19, Uzbekistan imposed strict lockdowns, implemented border control measures, and added 10,000 hospital beds in Tashkent to address the shortage of beds. From the beginning of the pandemic to late June, all confirmed cases in Uzbekistan were hospitalized to hospitals dedicated to COVID-19 even if they had no symptoms. This study aimed to investigate the symptoms and clinical characteristics of COVID-19 by age group and intensive care unit (ICU) group of Uzbekistan patients at the beginning of pandemic so that the findings of the study can be used to strengthen the response to COVID-19.
DISCUSSION
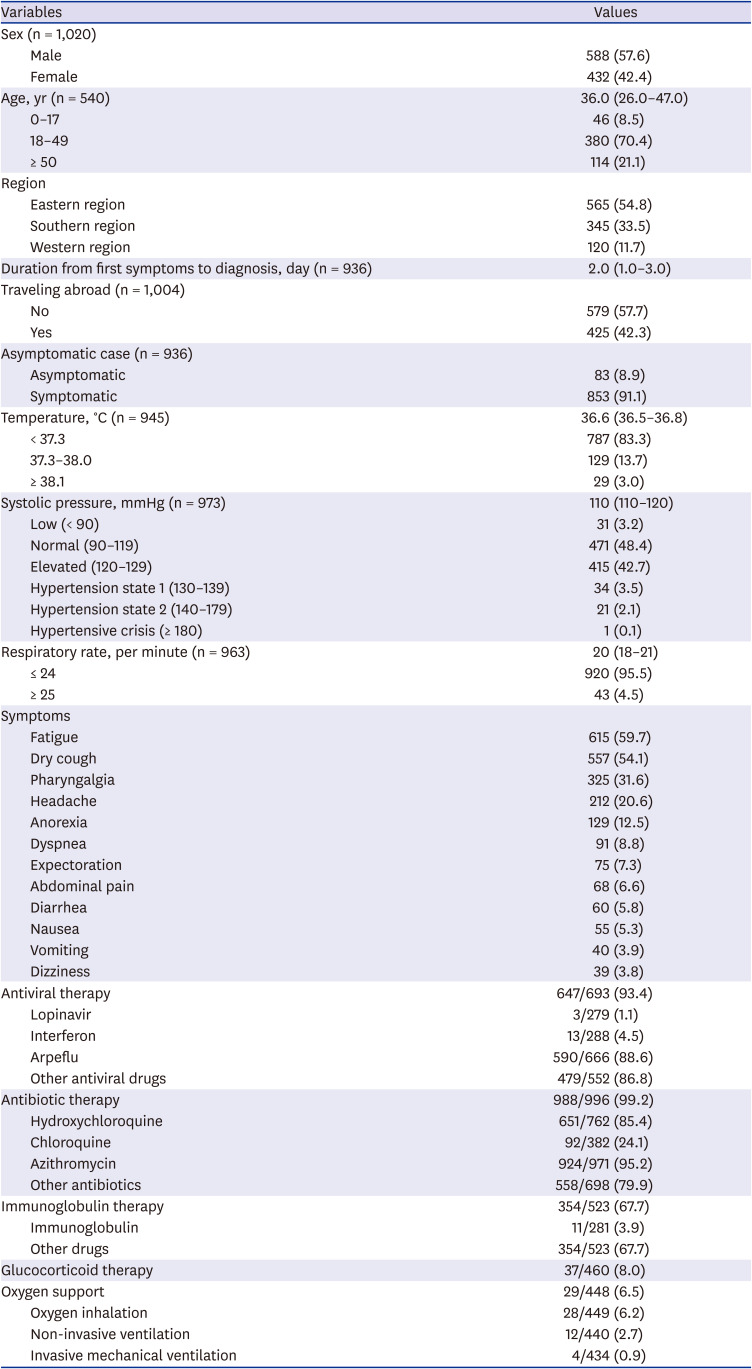
This study analyzed symptoms and clinical characteristics of confirmed COVID-19 patients admitted to COVID-19 hospitals in Uzbekistan. Therefore, this study is of considerable significance in that it analyzed the clinical characteristics by using representative data on a national scale in Uzbekistan.
In terms of the number of COVID-19 cases, 588 (57.6%) were male and 432 (42.4%) were female in this study. Many studies report that males are more vulnerable to COVID-19 than females. According to the United Nations Women, the portion of male (54.3%) was slightly higher than that of female (45.7%).
18 According to a retrospective study by Alsofayan et al.
19 in Saudi Arabia, 54.3% of the patients were male, and 45.7% were female. Argenziano et al.
20 also reported more male patients (59.6%) than females (40.4%). As a result of analyzing 7,755 confirmed cases of COVID-19 in Korea, there were more female (62.0%) than male (38.0%).
21
The mean age of COVID-19 cases in Uzbekistan was 37.0, while the median was 36 (IQR 26–47). Regarding the age group, 70.4% of the patients were aged 18–49 years, 21.1% were over 50 years, and 8.5% were under 17 years. In a study by Kim et al.,
22 the average age of the participants was 42.6 years. The median age of the COVID-19 cases in a study by Huang et al.
14 was 49 (IQR 41–58), indicating outbreaks of confirmed cases in the middle age group. In Korea, 6.2% of the confirmed cases were aged 0–19, 53.3% were aged 20–49, and 40.6% were aged 60 or older. WHO Europe urged for integrated and person-centered systems for long-term care facilities stressing that about half of COVID-19 mortality in European countries were those who live in long-term care facilities.
23 Nuclear and extended family cultures coexist in Uzbekistan, thus it is assumed that the risk of group outbreaks is relatively low among the elderly due to the lack of long-term care facilities.
24
In Uzbekistan, the median duration from symptom onset to diagnosis was 2 (IQR 1–3) days. In terms of the infection route, 42.3% of the confirmed cases came from abroad, indicating that a large number of confirmed cases from abroad caused a sharp increase in the early stages of the outbreak in Uzbekistan.
The eastern region, which includes Tashkent and Namangan, had the largest number of confirmed cases, accounting for more than half. Tashkent and Namangan have international airports, thus it is considered that people returning from foreign countries would be one of the routes for spreading the disease in the country. The decision to close their borders to stop the spread of COVID-19 by many countries has been controversial.
25 Gomes et al.
25 and Poletto et al.
26 reported that airline traffic reduction and travel restrictions delay the spread of infectious diseases. However, Gostin et al.
27 pointed out that strict travel restrictions are not scientifically effective in preventing the spread and would lead to undermine IHR in the long term. Nevertheless, although the aggressive quarantine policy in Uzbekistan was not able to completely block the spread of COVID-19 in the early stage, and it was successful in delaying the proliferation and preventing the overloading of the medical system in a short period. Thus, implementing social distancing was suitable for preventing local outbreaks in the country. According to the combination of COVID-19 data in Kazakhstan and susceptible-exposed-infected-removed modeling, Semenova et al.
10 showed the importance of social distancing by reporting that adherence to quarantine measures could decrease confirmed cases of COVID-19.
In Uzbekistan, 91.1% of COVID-19 cases were symptomatic. In a retrospective study of COVID-19 cases in Saudi Arabia, 90.7% of the cases were also symptomatic.
19
As a result of measuring the temperature of COVID-19 cases on admission in this study, 83.3% of the participants were within the normal range (< 37.3
°C). In this study, antipsychotic drugs were not used for patients with body temperature above 37°C, but if the temperature rose up to 38°C, it was recommended to take drugs containing paracetamol. Zhu et al.
28 reported that the most frequent symptom of 8,697 COVID-19 patients in China was fever (78.4%) through meta-analysis, which differed from the results of this study. According to temporary guidelines for COVID-19 patient management in Uzbekistan, it explains that the elderly and those with weak immune system may not experience fever, and the screening criteria includes travelling abroad within 14 days, close contacts with COVID-19 cases or those who have acute respiratory infection after visiting foreign countries, and one or more symptoms such as fever, cough, dry cough, or difficulty in breathing.
13 Thus, health care providers are recommended to consider asymptomatic characteristics of fever in the screening process. Fever has been reported as an essential clinical symptom of severe cases of COVID-19 in previous studies,
719 but the findings of this study suggest that screening issues based on fever may need to be adjusted in consideration of specific conditions and epidemiological characteristics.
In this study, 4.5% of the participants had a respiratory rate of more than 24 per minute. In another study, respiratory rate over 24 breaths per minute was higher than that of our study, and the reason can be inferred that it is related to the low severity and fatality rate of Uzbekistan COVID-19 cases.
14
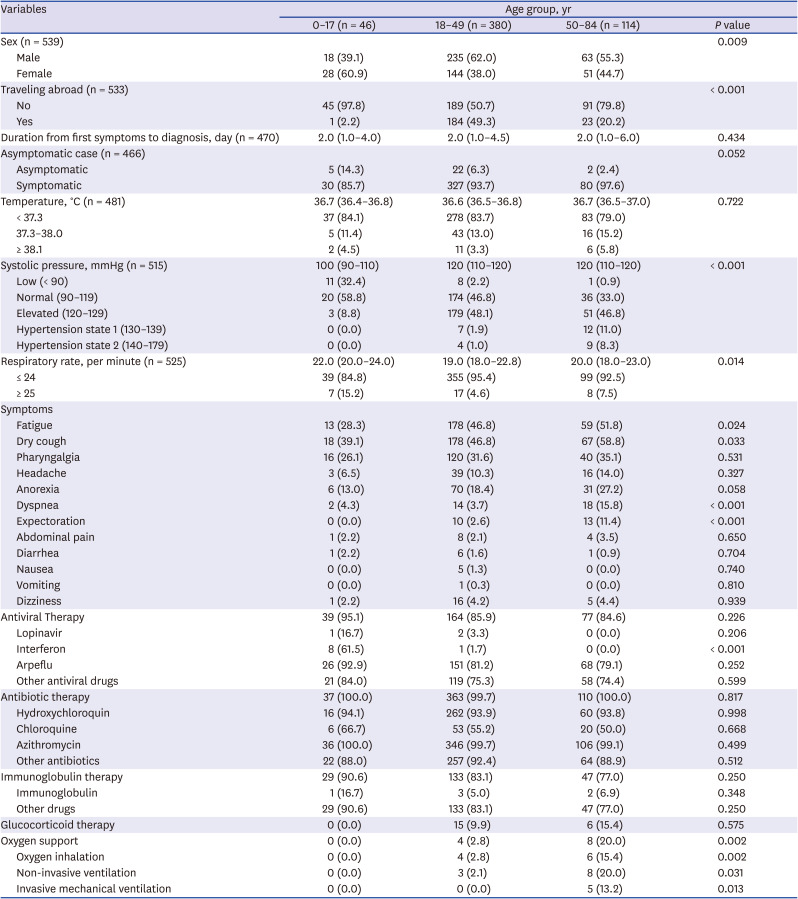
In this study, the main symptoms of the group aged 50–84 years were dry cough (58.8%), fatigue (51.8%), dyspnea (35.1%), and pharyngalgia (27.2%), and the main symptoms of the group aged 18–49 years were dry cough (46.0%), fatigue (46.0%), pharyngalgia (31.6%), anorexia (18.4%), and headache (15.8%). The main symptoms of participants aged 0–17 years were dry cough (39.1%), fatigue (28.3%), dyspnea (26.1%), pharyngalgia (13.0%), and abdominal pain (10.2%). Nonetheless, there were some differences in subjective symptoms. Zhu et al.
28 reported that the most frequent symptoms of COVID-19 patients in China were fever (78.4%), cough (58.3%), fatigue (34%), myalgia (21.9%), expectoration (23.7%), anorexia (22.9%), chest tightness (22.9%) and dyspnea (20.6%) through meta-analysis. Liu et al.
29 analyzed the risk factors associated with the severity of COVID-19, and the result showed that fatigue (odds ratio [OR], 3.74;
P = 0.054) was associated with severe cases. Among the COVID-19 cases in this study, 8.5% of the patients were aged under 18 years. According to a survey of the clinical and epidemiological characteristics of 36 children with COVID-19 in China, COVID-19 affected several vital organs with some amounts of myocardial enzymes, and a large number of asymptomatic cases in the pediatric group had the risk of community transmission.
7 In the US, the Centers for Disease Control and Prevention (CDC) reported that relatively few children under the age of 18 with COVID-19 were hospitalized, and showed fewer symptoms of fever, cough, or difficulty breathing than adults aged 18 to 64.
30
While most patients received antiviral and antibiotic therapy, and these findings were similar with a previous study,
8 oxygen support of this study was relatively low compared to a study conducted in Hubei Province, China.
15 The low severity might be explained in that patients were detected in the early stages and all confirmed patients were referred to the hospitals regardless of symptoms.
Among the different age groups of patients with COVID-19 in Uzbekistan, there were more female patients in group aged 0 to 17 years and more male patients in the rest of the groups. According to a researcher who participated in this study, it might be explained that parents of girls suspected of COVID-19 took them to the hospital immediately for fear of more complications than boys, and it led to prompt COVID-19 testing. With regard to traveling abroad, about half of the group aged 18–49 years reported that they came from abroad. This finding was similar with a study regarding initial characteristics of imported COVID-19 cases in Korea, given that the main age groups were 20–29, 30–39, 40–49.
31
As a result of comparing the temperature of each age group, the differences were not statistically significant. Though adults with COVID-19 showed a higher frequency of fever than children according to a clinician guide by Government of Canada,
32 circumstances in Uzbekistan were different. We can infer that the proportion of the elderly with high fever was relatively low, as temporary guidelines for COVID-19 management in Uzbekistan says that the elderly may not experience high temperature.
13 A study that analyzed the risk factors for elderly patients in Korea also found that the difference between surviving patients and deceased patients was not statistically significant.
33 Normal blood pressure (90–119 mmHg) were statistically different between the groups.
The blood pressure presented in this study is the result measured on admission, rather than underlying diseases such as the high blood pressure of the patients. According to a survey of patients over 65 years old in Korea, the mean blood pressure of surviving and deceased patients was 138.1 mmHg and 114.0 mmHg, respectively, and the difference between the groups was statistically significant.
33
Although underlying diseases were not included in this study, Liu et al.
29 reported that disease severity and hypertension were highly associated (OR, 3.59;
P = 0.048). However, a study by Lee et al.
33 reported that blood pressure was not statistically significant as a risk factor for underlying diseases in patients over 65 years old.
The respiratory rate between three age groups in this study was statistically significant. Compared to older groups, patients aged 0 to 17 years had a higher portion of more than 24 respiratory rate per minute. In can be inferred that the infant and toddler's normal respiratory rate is higher than that of adults, and it led to the highest percentage of respiratory rate over 24 in the youngest group.
3435
Symptoms showed statistical differences between age groups were fatigue, dry cough, anorexia, dyspnea, and expectoration, and the symptoms were most frequent in the oldest group. According to a study on different influence of COVID-19 by age, children with COVID-19 showed asymptomatic or mild symptoms compared to adults.
36
Regarding the therapies and treatment, only oxygen support showed significant differences. While no patients in group aged 0–17 years received oxygen support, one-fifth of patients in group 50–84 received the support. This result is similar to a study of 3,060 COVID-19 patients, in which patients under the age of 50 were able to recover without oxygen support.
37
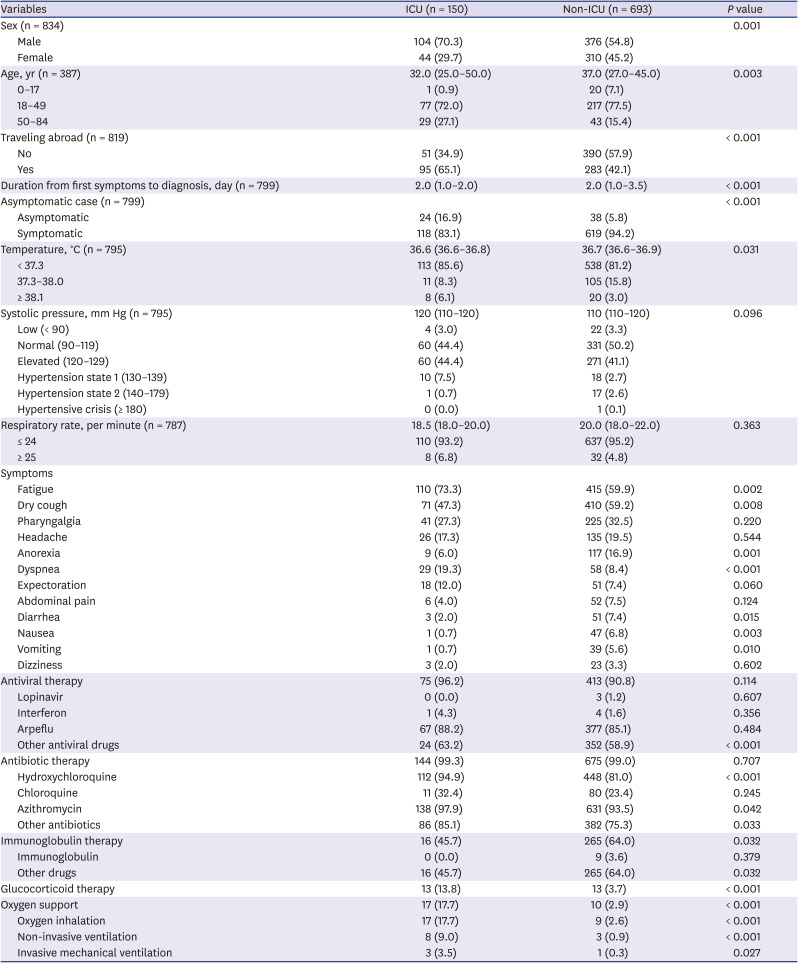
Comparing ICU and non-ICU patients, more male patients (70.3%) were admitted to ICU than female (29.7%), which was consistent with a clinical study conducted in Wuhan, China.
14 The portion of patients aged 50–84 years of ICU group was higher than that of those who were not admitted to ICU. In a comparative COVID-19 study of clinical characteristics by severity, 69.1% of patients aged more or 65 showed high severity scores ranging from 5 to 8.
37 Compared to non-ICU group, patients in ICU group had more international travel histories. According to GISAID, COVID-19 are classified as S, L, V, G, GH and GR clades by nucleic acid sequence,
38 Korea Centers for Disease Control and Prevention (KCDC) announced that clades can be varied according to assumed country of origin.
39 KCDC analyzed virus classification and found epidemiological information with specific clade: S clade were identified from imported cases during early period and GR were found from crews in Russian ship arrived at Busan port or passengers of international arrivals.
40 Analysis of the nucleic acid sequence in Uzbekistan does not exist as far as we know, thus further study needs to be conducted to analyze relationships between epidemiological information and virus classification.
While duration from the first symptoms to diagnosis was significantly different between two groups in this study, a study regarding 3,060 patients with COVID-19 in Korea showed that there was no statistically significant differences between COVID-19 severity scores.
37 There were more asymptomatic patients in ICU group than non-ICU group. In a study related to asymptomatic infections with COVID-19 in China, asymptomatic transmission within family members showed severe pneumonia.
41
Body temperature between ICU and non-ICU group were significantly different. In this study, there were more ICU cases with high temperature over 38.1°C, compared to non-ICU cases. Similarly, a study analyzing the characteristics of patients in a university hospital in Daegu, Korea showed the differences of temperature between severe and non-severe group, and high body temperature was found to affect the severity of hospitalized COVID-19 patients.
8
In this study, the median blood pressure of the ICU group was higher than that of the non-ICU group, which was similar to a survey by Huang et al.
14 However, there was no statistically significant difference in blood pressure between the groups in this study. According to a study of inpatients in a university hospital in Daegu, Korea, the mean blood pressures of severe and non-severe group was 134.9 and 126.3 mmHg, respectively, but there was no statistical significance.
8 In a study by Huang et al.,
14 there were statistically significant differences between the blood pressure and respiratory rate per minute between the ICU and non-ICU groups.
22
The respiratory rate between the ICU and non-ICU group in this study was not statistically significant, but the statistical differences between the two groups were significant in the studies on patients in Hubei Province, China, and patients in a university hospital in Daegu, Korea.
815 According to a study comparing the clinical characteristics between a severe and conventional group by Tian et al.,
42 the difference between the mean temperature and respiratory rate of the two groups was not statistically significant.
Different symptoms in between the ICU and non-ICU group were fatigue, dry cough, anorexia, dyspnea, diarrhea, nausea and vomiting. In a study to compare clinical characteristics between deceased and survived patients with COVID-19, symptoms that exhibited significant differences between the two groups were sputum, dyspnea, febrile sensation and diarrhea.
43 ICU group received less immunoglobulin therapy, more glucocorticoid therapy and oxygen support than non-ICU group. Antiviral therapy, antibiotics, pegylated interferon-α, oxygen supplement were used more for deceased patients than survivors of COVID-19 in a study analyzing COVID-19 patients hospitalized at nationally-designated treatment hospitals in Korea.
43
This study has limitations in data analysis due to missing values in age and clinical information about study participants. The clinical symptoms were measured on admission to specialized hospitals after confirmation of COVID-19. Thus, this study lacks an in-depth analysis of the progression of COVID-19 patients. Nevertheless, as this study has investigated patients in hospitals specialized in infectious diseases across Uzbekistan, the contents can be used as objective evidence to implement policies related to COVID-19 in Uzbekistan.
In conclusion, demographic and clinical characteristics were different according to age and ICU group in this study. The oldest group showed more symptoms than the youngest one, and the proportion of asymptomatic cases and visiting abroad in ICU group were more than those of in non-ICU group. Thus, the medical delivery system and resource distribution need to be implemented based on clinical characteristics by age and severity so that it can respond effectively to COVID-19 and delay the spread. Moreover, early detection to find asymptomatic cases of those who returned from abroad need to be set up at the beginning of the pandemic.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download