Praziquantel is active against a broad range of parasitic helminths, including clonorchiasis, schistosomiasis, opisthorchiasis, tapeworm infections, cysticercosis and hydatid disease.
1 Praziquantel is mainly used to treat clonorchiasis in Korea, but it is widely used worldwide as a treatment for schistosomiasis. Schistosomiasis is endemic in 78 countries and is a major public health problem in many tropical countries—particularly in sub-Saharan Africa.
2 Schistosomiasis is not endemic in Korea and therefore, only the imported cases have been reported in travelers to endemic areas of schistosomiasis.
3 The strategy for schistosomiasis control aims to prevent morbidity in later life through regular treatment with praziquantel, which is currently the only recommended drug for curing schistosome infections.
45 A number of international agencies, notably the United Nations International Children's Fund and the World Health Organization (WHO),
6 along with for-profit and non-profit organizations, supply praziquantel in bulk to developing countries. Although different brands of praziquantel have been widely used in Africa for controlling schistosomiasis, information regarding the effectiveness of each brand is scant.
78 Therefore, this study aimed to compare the anthelminthic effects of three different commercial brands of praziquantel among school children treated for
Schistosoma haematobium infection.
Schistosomiasis, particularly
S. haematobium infection, remains a major public health concern in Sudan.
910 To control schistosomiasis in Sudan, the authors participated an integrated control program including mass chemotherapy with praziquantel from 2009 until 2019 by the support of the Korea International Cooperation Agency. This study was conducted from January 2018 to March 2018 in the White Nile State of Sudan. We prepared three commercial brands of praziquantel: Distocide
® General Medicines Company, Khartoum, Sudan; Epiquantel
® Egyptian International Pharmaceutical Industries Co., Ramadan City, Egypt; and Praziquantel
® Merck & Co., Inc., Darmstadt, Germany. These brands of praziquantel, including Merck's, have no differences in the chemical structure and composition (C
19H
24N
2O
2).
Children from six primary schools in White Nile State of Sudan (Al Dobasi, Al Hidaib, Al Naeem, Al Marabea, Al Salam, Al Sefeira) were randomly allocated to three treatment groups, with each group assigned one brand of praziquantel from three different companies. In order to manage each cohort, 1,286 children (average age 8.6 years; age range of 6–16 years; and boys to girls ratio of about 3:1) from the first to third grades of each school received a specific identity number (ID). We excluded children who were either seriously ill or were allergic to praziquantel, or who had taken medication for schistosomiasis within four weeks from the time of the baseline survey.
Urine samples were collected from schoolchildren at the baseline survey and two weeks after treatment (follow-up survey). The samples were screened by microscopy for
S. haematobium eggs after centrifugation of 10 mL.
910 Children were orally administered one brand of praziquantel tablets at a dose of 40 mg/kg using a dose pole.
2 Infection intensities of
S. haematobium are expressed as geometric mean intensity of eggs per 10 mL of urine. For quality control, 10% of slides were randomly selected and re-examined by parasitology experts.
Three different commercial brands of praziquantel were randomly categorized as brands A, B, and C for the double-blind test. The cure rates were analyzed on the basis of the intention-to-treat analysis principle. Generalized estimating equation was used for assessing relative risk (RR) as well as 95% confidence intervals (CIs) of schistosomiasis prevalence after treatment with brand A or B compared to C. Multi-level mixed linear regression was performed to assess the mean difference (MD) and 95% CIs of infection intensity of schistosomiasis between the group A or B and C.
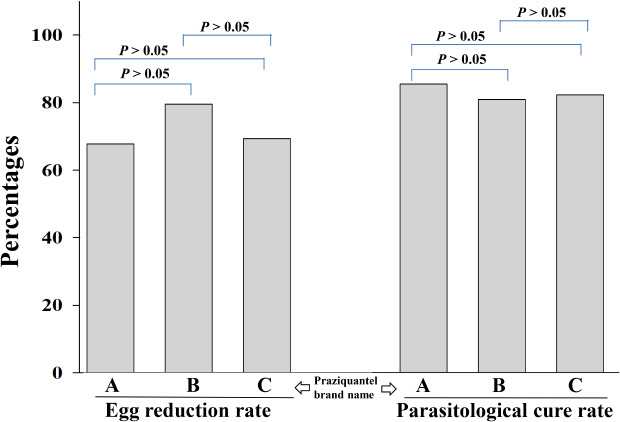
The baseline prevalence rates of
S. haematobium infection in the groups A, B, and C were 14.8%, 15.8%, and 15.6%, respectively. For the same groups, the prevalence rates at two weeks post-treatment were reduced to 4.8%, 5.2%, and 5.8%, respectively (
Table 1), while the egg-reduction rates were 69.0%, 81.0%, and 70.6%, respectively (
Table 2). There was no statistically significant difference in the reduction of prevalence rates (group A: RR, 0.83, 95% CI, 0.46–1.48,
P = 0.52; group B: RR, 0.69, 95% CI, 0.43–1.10,
P = 0.12) and egg-reduction rates (group A: MD, 5.56, 95% CI, −1.02–12.13,
P = 0.10; group B: MD, 1.52, 95% CI, −2.68–5.73,
P = 0.48) of group A and B compared to those of group C (
Tables 1 and
2). Two weeks after the treatment, the cure rates of
S. haematobium-infected children were 87.1%, 82.4%, and 83.8% in groups A, B, and C, respectively (
Table 3). There was no statistical significance in the anthelminthic effects of brands A, B, and C praziquantel (group A: RR, 1.03, 95% CI, 0.99–1.07,
P = 0.12; group B: RR, 1.0, 95% CI, 0.95–1.05,
P = 0.98).
Table 1
Effect of treatment A and B on the prevalence of Schistosoma haematobium infection among school children compared with treatment C at 2 weeks after praziquantel treatment

|
Treatment groups |
Baseline, % (No.) |
Post-treatment, % (No.) |
RR |
95% CI |
P value |
|
C |
15.60 (80/513) |
5.82 (26/447) |
Reference |
|
|
|
A |
14.80 (47/318) |
4.85 (11/227) |
0.83 |
0.46–1.48 |
0.52 |
|
B |
15.80 (72/455) |
5.15 (10/194) |
0.69 |
0.43–1.10 |
0.12 |

Table 2
Effect of treatment A and B on the intensity of Schistosoma haematobium-infected children compared with treatment C at 2 weeks after praziquantel treatment

|
Treatment groups |
Baseline, mean (SE) |
Post-treatment, mean (SE) |
MD |
95% CI |
P value |
|
C |
17.25 (26.15)a
|
5.07 (6.18)a
|
Reference |
|
|
|
A |
34.30 (43.58) |
10.64 (15.04) |
5.56 |
−1.02, –12.13 |
0.10 |
|
B |
34.72 (49.99) |
6.60 (5.21) |
1.52 |
−2.68, –5.73 |
0.48 |

Table 3
Effect of treatment A and B on parasitological cure rate of Schistosoma haematobium-infected children compared with treatment C at 2 weeks after praziquantel treatment

|
Treatment groups |
Baseline, positive, No. |
Post-treatment, % (No.) |
RR |
95% CI |
P value |
|
C |
80 |
83.8a (57/68) |
Reference |
|
|
|
A |
47 |
87.1 (27/31) |
1.03 |
0.99–1.07 |
0.12 |
|
B |
72 |
82.4 (28/34) |
1.00 |
0.95–1.05 |
0.98 |

WHO declared that all children would get access to universal preventive chemotherapy in collaboration with Merck, the primary producer of praziquantel. In 2007, Merck committed to donating praziquantel to children in need across the world. The commitment enabled the treatment of 400 million school-aged children in sub-Saharan Africa since 2007. Even in 2019, they renewed commitment to their Praziquantel Donation Progarm.
11 Since the other products of praziquantel are not provided free of charge, we can hardly argue the economic advantage of these non-Merck praziquantels. However, there are much more meaningful implications of the findings in this study, as follows. In spite of the worldwide donation program by Merck, some areas still lack access to Merck's praziquantel due to various reasons including logistical difficulties in its delivery to some remote areas. In addition, adults who may require this therapy are not guaranteed access to it. Because of this lack of supply of the Merck brand, in reality, other brands are being used in many parts of sub-Saharan Africa. If the non-Merck products proved less effective, it must draw strong attention from the global community. Therefore, it is important to ascertain whether the various praziquantel brands available on the market are comparable in terms of effectiveness. Fortunately, this study suggests that the non-Merck products are not less effective compared with the Merck products. However, we acknowledge the non-Merck products should be used tentatively as long as they are sold, not donated, and Merck should make efforts for universal access to every corner around the world since the people in need are mainly the most vulnerable people, who cannot afford to buy the products.
In this study, the prevalence rate of
S. haematobium infection was reduced to 62.7%–67.4% at 2 weeks after treatment, which was similar to the prevalence rate of 57.8% reported in a previous study from South Africa.
12 The cure rates of the three brands in our study ranged between 82.4% and 87.1%, while it was 57.9% and 84.6% in South Africa
1213 and 86.2% in Kenya.
14 The egg reduction rates in this study ranged between 64.2% and 72.4% at two weeks post-treatment, while it was reported as 64% and 80% in males and females, respectively, four weeks later.
12 According to one meta-analysis, the cure rate and egg-reduction rate of 40 mg/kg praziquantel for
S. haematobium in schoolchildren were 71.4% and 94.4%, respectively.
5 The reported cure rates and egg-reduction rates have a wide range, and the present findings of the three brands fall within this range. We found no complaints of adverse effects of the medication, which may have had something to do with taking the medication post breakfast.
A major limitation of the study was the short follow-up period of two weeks. Besides, there was just one check-up. The short follow-up duration may have affected the results because the eggs may have hatched in the tissue.
1215 Further, for this study, we had to use data collected along with routine monitoring activities of schistosomiasis control project in Sudan. For this reason, we could not design a rigorous randomized control trial. Specifically, we believed that the randomized control trial at the individual level, as opposed to at the cluster level, would be a more appropriate study design but some stakeholders did not approve of distributing different brands of praziquantel in the same school. In addition, because of the limited budget, we could not enroll sufficient number of clusters. Nonetheless, despite these limitations, the study was able to demonstrate that the three brands of praziquantel being used in Sudan have similar anthelminthic effects against urogenital schistosomiasis.
Ethics Statement
The study protocol was reviewed and approved by the Institutional Review Board (IRB) of the Korea Association of Health Promotion (KAHP) (IRB approval No. 130750–20,164-HR-020) and Federal Ministry of Health, Sudan (FMOH/DGP/RD/TC/2016). We also obtained verbal informed consent from each child in the presence of the school teachers.
Go to :








 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download