INTRODUCTION
Sjögren's syndrome (SS) is a chronic autoimmune disease that involves exocrine glands with inflammatory infiltrates and leads to hypo-function of the salivary and lacrimal glands. One third of patients present with fatigue, arthralgia, myalgia, depression, or systemic extra-glandular inflammatory involvement of the lung, musculoskeletal, or neurologic system.
1 SS patients also have a higher risk of other comorbidities, including non-Hodgkin lymphoma, compared with the general population.
2
SS is closely associated with other autoimmune rheumatic diseases, such as systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA). In previous studies, the prevalence of SS in SLE patients ranged from 9% and 19%.
345 The prevalence of SS among RA patients has been reported to be as high as 55%.
6 Those reports estimated prevalence using rheumatologists' diagnoses, the 1993 European Classification criteria, and the 2002 American-European Consensus Group (AECG) classification criteria for SS. Discrepancies in the prevalence of SS among RA patients were caused by the use of different classification criteria for diagnosis.
Previous studies indicated that the coexistence of RA and SS could result in more severe arthritis with worse joint damage than is typical with RA alone,
7 as well as a higher rate of systemic involvement through anemia and lung function changes.
8 Other studies have shown that having both SS and RA increased the risk of non-Hodgkin's lymphoma and higher mortality compared to those with RA alone.
910 However, no research has adequately examined the prevalence and clinical effects of SS on RA disease activity and treatment. Indeed, there have been insufficient studies on the prevalence of disease-specific autoantibodies among RA patients with SS, even though the positivity of autoantibody profiles of primary SS patients were already investigated in other studies.
1112
Therefore, we aimed to estimate the prevalence of SS in RA patients according to various classification criteria for SS as well as specific autoantibodies and to compare the clinical features and treatment patterns of RA patients with and without SS.
METHODS
Study population
We conducted a retrospective study of RA patients who visited a rheumatology clinic in a tertiary referral hospital in Korea between May 20 and July 22, 2016. They were all adult patients aged over 20 and met the 1987 American College of Rheumatology (ACR) or 2010 ACR/European League Against Rheumatism (EULAR) classification criteria for RA. In addition, all RA patients who visited the clinic during the study period had previously been screened for SS by asking about the presence of any sicca symptoms. If they agreed, patients who reported sicca symptoms received diagnostic tests for SS (a screen for autoantibodies, labial salivary gland biopsy, salivary scintigraphy, Schirmer's test, or ocular staining) prior to the registration period. Subject medical records were reviewed retrospectively to determine the presence of SS as judged by clinical impressions, clinical symptoms, and diagnostic tests.
Prevalence of SS in RA patients
The prevalence of SS among RA patients was first estimated using rheumatologists' clinical diagnoses. Rheumatologists diagnosed SS in patients with compatible symptoms or signs of SS and results from SS diagnostic tests. Then, the patients the rheumatologists diagnosed with SS were reassessed to determine whether they fulfilled the 2002 AECG,
1 2012 ACR,
13 or 2016 ACR/EULAR classification criteria.
14 We also estimated the prevalence of SS in RA patients who reported any sicca symptoms.
As subgroup analyses, we estimated the prevalence of SS in RA patients with rheumatoid factor (RF) antibodies, anti-nuclear antibodies (ANAs), or anti-Ro antibodies.
Clinical characteristics of RA patients with SS
The clinical characteristics and treatment patterns of RA patients with and without SS were compared. RA disease activity was assessed using the Disease Activity Score 28 (DAS28)-erythrocyte sedimentation rate (ESR), and laboratory findings for RF, anti-cyclic citrullinated peptide antibody, ESR, and C-reactive protein. The treatment patterns for RA were investigated in terms of the medications prescribed to control disease activity, including conventional synthetic and biologic disease modifying antirheumatic drugs (DMARDs).
Statistical analysis
The prevalence of SS was estimated as the number of RA patients who were diagnosed with SS within the total number of RA patients. We present the clinical characteristics of patients as the median with interquartile range (IQR) for continuous variables and as numbers with percentages (%) for categorical variables. We compared the clinical characteristics of RA between patients with and without SS using independent t-tests or χ2 tests. Any P value less than 0.05 was regarded as statistically significant. All statistical analyses were performed with the Statistical Package for Social Sciences (SPSS) version 24.0 for Windows (SPSS Inc., Chicago, IL, USA).
Ethics statement
This study was approved by the Institutional Review Board of Hanyang University Hospital (HYUH 2016-03-040-021), and the need for informed consent was waived because the data were de-identified and collected retrospectively.
RESULTS
Baseline characteristics of the study population
We included 827 RA patients in this study (
Fig. 1), and their overall clinical characteristics are provided in
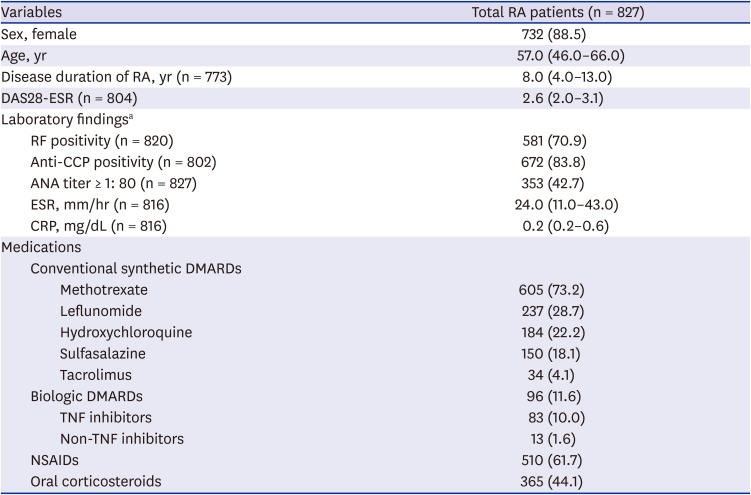
Table 1. Most RA patients were female (88.5%) and their median age was 52 years old, and the median duration of RA was 8.0 years (IQR 4.0–13.0). Their RA disease activity, estimated by median DAS28-ESR, was 2.6 (IQR 2.0–3.1). In terms of their treatments, methotrexate was the most frequently used DMARD (73.2%), followed by leflunomide (28.7%) and hydroxychloroquine (22.2%). Biologic DMARDs (bDMARDs) were used in 11.6% of RA patients, and most of them (86.5%) were tumor necrosis factor inhibitors.
Fig. 1
Patient selection flow for patients with RA and coexisting SS.
RA = rheumatoid arthritis, SS = Sjögren's syndrome, ACR = American College of Rheumatology, EULAR = European League Against Rheumatism.


Table 1
Baseline characteristics of all RA patients in this study
|
Variables |
Total RA patients (n = 827) |
|
Sex, female |
732 (88.5) |
|
Age, yr |
57.0 (46.0–66.0) |
|
Disease duration of RA, yr (n = 773) |
8.0 (4.0–13.0) |
|
DAS28-ESR (n = 804) |
2.6 (2.0–3.1) |
|
Laboratory findingsa
|
|
|
RF positivity (n = 820) |
581 (70.9) |
|
Anti-CCP positivity (n = 802) |
672 (83.8) |
|
ANA titer ≥ 1: 80 (n = 827) |
353 (42.7) |
|
ESR, mm/hr (n = 816) |
24.0 (11.0–43.0) |
|
CRP, mg/dL (n = 816) |
0.2 (0.2–0.6) |
|
Medications |
|
|
Conventional synthetic DMARDs |
|
|
|
Methotrexate |
605 (73.2) |
|
|
Leflunomide |
237 (28.7) |
|
|
Hydroxychloroquine |
184 (22.2) |
|
|
Sulfasalazine |
150 (18.1) |
|
|
Tacrolimus |
34 (4.1) |
|
Biologic DMARDs |
96 (11.6) |
|
|
TNF inhibitors |
83 (10.0) |
|
|
Non-TNF inhibitors |
13 (1.6) |
|
NSAIDs |
510 (61.7) |
|
Oral corticosteroids |
365 (44.1) |

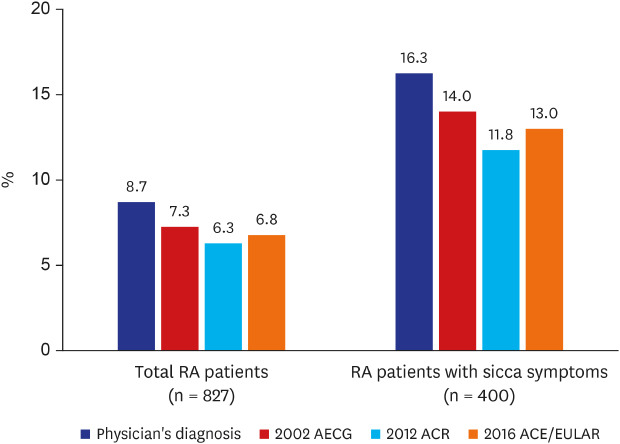
Prevalence of SS in RA patients
Among the 827 RA patients, 72 patients (8.7%) were diagnosed with SS by rheumatologists (
Fig. 2). Diagnostic tests for SS were suggested to all 72 patients, but they were only performed with patient agreement; minor salivary gland biopsy and salivary gland scintigraphy were performed in 57 and 66 patients, respectively (
Table 2). Ocular staining, Schirmer's test, and the tear breakup test were performed in 58, 57, and 49 patients, respectively, with ocular symptoms. Sixty of the 827 RA patients (7.3%) satisfied the 2002 AECG classification criteria for SS. Fifty-two patients (6.3%) met the 2012 ACR classification criteria, and 56 patients (6.8%) fulfilled the 2016 ACR/EULAR classification criteria. The prevalence of SS tended to be higher among RA patients with sicca symptoms (
Fig. 2). All the patients who received a salivary gland biopsy (n = 57) presented histologic findings compatible with SS (
Table 2). Functional impairment of the salivary glands was found in 57 of the 66 patients who received the salivary gland scintigraphy test (86.4%).
Fig. 2
Prevalence of Sjögren's syndrome in RA patients, overall and with sicca symptoms.
RA = rheumatoid arthritis, AECG = American-European Consensus Group, ACR = American College of Rheumatology, EULAR = European League Against Rheumatism.
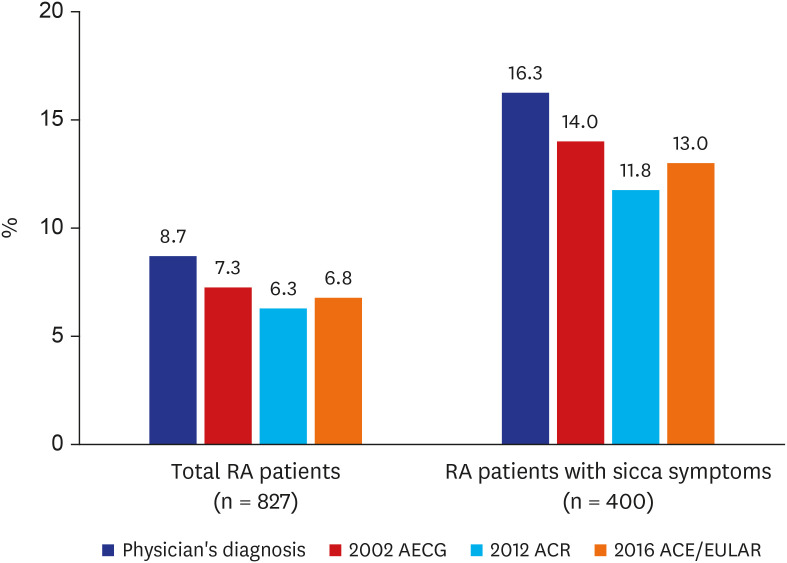

Table 2
Clinical characteristics of RA patients with SS

|
Variables |
RA with SS (n = 72) |
|
No. of patients satisfying diagnostic criteria for SS |
|
|
Rheumatologists' clinical diagnosis |
72 (100.0) |
|
2002 AECG criteria |
60 (83.3) |
|
2012 ACR classification criteria |
52 (72.2) |
|
2016 ACR/EULAR criteria |
56 (77.8) |
|
Glandular features |
|
|
Dry eye |
56 (77.8) |
|
Foreign body sensation in eye |
12 (16.7) |
|
Dry mouth |
49 (68.1) |
|
Objective gland functions |
|
|
No. of patients with ocular staining score ≥ 5 (n = 58)a
|
22 (37.9) |
|
No. of patients with abnormal Schirmer's test (n = 57)a
|
24 (42.1) |
|
No. of patients with abnormal TBUT (n = 49)a
|
47 (95.9) |
|
|
TBUT, right |
3.0 (2.0–3.5) |
|
|
TBUT, left |
3.0 (2.0–4.0) |
|
No. of patients with focus score ≥ 1 in salivary gland biopsy (n = 57)a
|
57 (100) |
|
No. of patients with abnormal salivary gland scintigraphy (n = 66)a
|
57 (86.4) |

Prevalence of SS in RA patients with specific antibodies
RF-positive RA patients
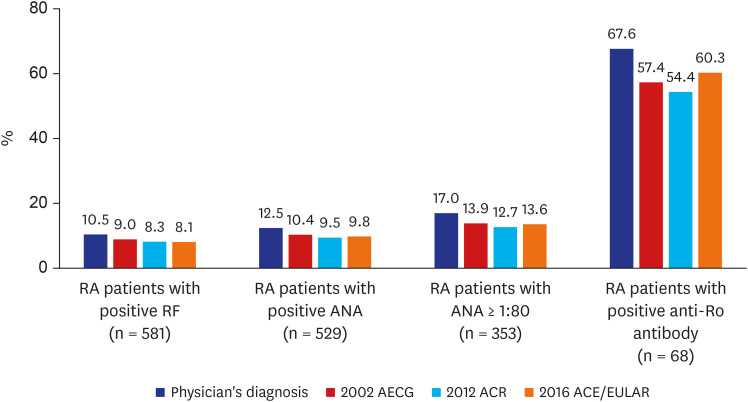
Most of the 820 RA patients were tested for RF, and 581 patients showed a positive result. Among the RF-positive RA patients (n = 581), 61 patients (10.5%) had rheumatologist-diagnosed SS (
Fig. 3). Among the RF-negative RA patients (n = 239), only 11 patients (4.6%) had rheumatologist-diagnosed SS. According to the 2002 AECG classification criteria, 52 RF-positive patients (9.0%) had SS, and 48 (8.3%) and 47 RF-positive patients (8.1%) were diagnosed with SS based on the 2012 ACR and 2016 ACR/EULAR classification criteria, respectively.
Fig. 3
Prevalence of Sjögren's syndrome in RA patients positive for specific autoantibodies.
RA = rheumatoid arthritis, AECG = American-European Consensus Group, ACR = American College of Rheumatology, EULAR = European League Against Rheumatism, RF = rheumatoid factor, ANA = anti-nuclear antibody.

ANA-positive RA patients
Of the 827 RA patients, 686 received the ANA serologic test, and positive results were documented in 529 of them (77.1%) (
Table 3). Among the 353 RA patients with ANA titers greater than or equal to 1:80, 60 (17.0%) had rheumatologist-diagnosed SS, and 49 (13.9%), 45 (12.7%), and 48 ANA-positive RA patients (13.6%) met the 2002 AECG, 2012 ACR, and 2016 ACR/EULAR classification criteria for SS, respectively (
Fig. 3).
Table 3
Comparison of clinical characteristics and treatment patterns of RA according to the presence of SS
|
Variables |
RA with SS (n = 72) |
RA without SS (n = 755) |
P value |
|
Sex, female |
69 (95.8) |
553 (80.2) |
0.410 |
|
Age, yr |
52.0 (43.0–60.8) |
57.0 (46.0–66.0) |
0.005 |
|
Comorbidity |
|
|
|
|
Hypertension |
9 (12.5) |
183 (24.2) |
0.024 |
|
Diabetes mellitus without complication |
1 (1.4) |
70 (9.3) |
0.023 |
|
Interstitial lung disease |
1 (1.4) |
33 (4.4) |
0.352 |
|
Cancer |
3 (4.2) |
16 (2.1) |
0.268 |
|
Lymphoma |
0 (0.0) |
1 (0.1) |
NC |
|
Other |
19 (26.4) |
244 (29.5) |
0.302 |
|
Sicca symptoms |
|
|
|
|
Dry mouth (n = 72, 724)a
|
49 (68.1) |
179 (24.7) |
< 0.001 |
|
Dry eye (n = 72, 724)a
|
56 (77.8) |
247 (34.1) |
< 0.001 |
|
Foreign body sensation in eye (n = 72, 724)a
|
12 (16.7) |
42 (5.8) |
< 0.001 |
|
Disease duration of RA, yr (n = 70, 703)a
|
7.5 (3.0–14.0) |
8.0 (4.0–13.0) |
0.448 |
|
DAS28-ESR (n = 72, 732)a
|
2.8 (2.3–3.2) |
2.6 (2.0–3.1) |
0.050 |
|
Laboratory findings |
|
|
|
|
ANA positivity (n = 72, 614)a
|
66 (91.7) |
463 (75.4) |
0.002 |
|
|
ANA titer ≥ 1:80 (n = 72, 614)a
|
60 (83.3) |
293 (47.7) |
< 0.001 |
|
Anti-Ro antibody positivity (n = 72, 425)a
|
46 (63.9) |
22 (5.2) |
< 0.001 |
|
Anti-La antibody positivity (n = 72, 425)a
|
24 (33.3) |
7 (1.6) |
< 0.001 |
|
|
RF positivity (n = 72, 748)a
|
61 (84.7) |
520 (69.5) |
0.007 |
|
|
Anti-CCP positivity (n = 72, 730)a
|
66 (91.7) |
606 (83.0) |
0.057 |
|
|
ESR, mm/hr (n = 72, 744)a
|
31.0 (16.0–49.0) |
23.0 (11.0–42.0) |
0.030 |
|
|
CRP, mg/dL (n = 72, 744)a
|
0.2 (0.2–0.7) |
0.2 (0.2–0.6) |
0.966 |
|
Medications |
|
|
|
|
Conventional synthetic DMARDs |
58 (80.6) |
547 (72.5) |
0.138 |
|
|
Methotrexate |
23 (31.9) |
214 (28.3) |
0.519 |
|
|
Leflunomide |
21 (29.2) |
163 (21.6) |
0.140 |
|
|
Hydroxychloroquine |
15 (20.8) |
135 (17.9) |
0.535 |
|
|
Sulfasalazine |
3 (4.2) |
31 (4.1) |
0.980 |
|
|
Tacrolimus |
4 (5.6) |
92 (12.2) |
0.093 |
|
Biologic DMARDs |
|
|
|
|
|
TNF inhibitors |
4 (5.6) |
79 (10.5) |
0.185 |
|
|
Non-TNF inhibitors |
0 (0) |
13 (1.7) |
NC |
|
NSAIDs |
46 (63.9) |
464 (61.5) |
0.685 |
|
Oral corticosteroids |
37 (51.4) |
328 (43.4) |
0.195 |

Anti-Ro antibody–positive RA patients
Among the 529 patients with positive ANA test results, 497 RA patients were tested for the anti-Ro antibody, and 68 (13.7%) patients tested positive. Among them, 46 (67.6%), 39 (57.4%), 37 (54.4%), and 41 (60.3%) patients (19.2%) were diagnosed with SS by a rheumatologist or the 2002 AECG, 2012 ACR, and 2016 ACR/EULAR classification criteria, respectively (
Fig. 2). The other 22 RA patients who were positive for the anti-Ro antibody were not diagnosed with SS by rheumatologists.
Clinical characteristics of RA according to the presence of SS
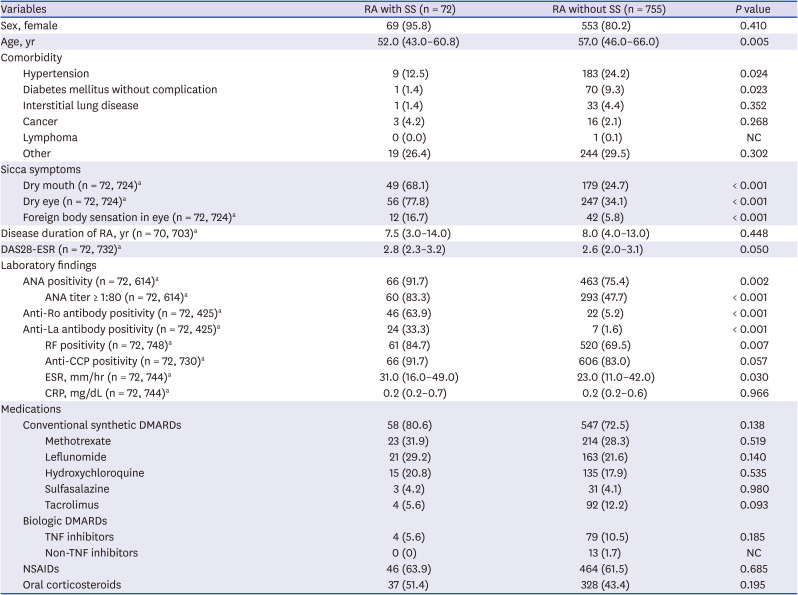
Most RA patients were female, and the proportion of female patients did not differ among those with and without SS. Patients who had both RA and SS were younger than those without SS (median 52.0 [IQR 43.0–60.8] vs. 57.0 [IQR 46.0–66.0], P = 0.005). With regards to comorbidities, the prevalence of hypertension and diabetes mellitus was lower in the RA patients with SS than in those without SS (12.5% vs. 24.2%, P = 0.024 and 1.4% vs. 9.3%, P = 0.023, respectively).
RF-positivity was more common in RA patients with SS than in those without SS (84.7% vs. 69.5%, P = 0.007), and the prevalence of ANA (titers greater than or equal to 1:80) was also higher in RA patients with SS than in those without SS (83.3% vs. 47.7%, P < 0.001). Autoantibodies, the anti-Ro antibody (63.9% vs. 5.2%, P < 0.001) and anti-La antibody (33.3% vs. 1.6%, P = 0.023), were more frequently detected in RA patients with SS than in those without SS.
Sicca symptoms were more prevalent in RA patients with SS (90.3% vs. 46.3%, P < 0.001), and the most frequent sicca symptom was dry eye (77.8% vs. 34.1%, P < 0.001), followed by dry mouth (68.1% vs. 24.7%, P < 0.001), and foreign body sensation in the eye (16.7% vs. 5.8%, P < 0.001). The use of bDMARDs was more frequent in patients without SS, whereas the use of conventional synthetic DMARDs, oral corticosteroids, and non-steroidal anti-inflammatory drugs was more frequent in those with SS. However, the overall treatment patterns for RA did not differ according to the presence of SS.
DISCUSSION
We estimated the prevalence of SS among 827 RA patients and found it to be 8.7% by rheumatologist diagnosis. Among those diagnosed by a rheumatologist, 60 patients (7.3%) satisfied the 2002 AECG classification criteria for SS, and 52 (6.3%) and 56 patients (6.8%) fulfilled the 2012 ACR and 2016 ACR/EULAR classification criteria, respectively. RA patients with SS had a higher rate of disease-specific autoantibodies (RF, ANA, and anti-Ro antibodies) than those without SS. Their mean age was younger, and they had a lower prevalence of hypertension and diabetes mellitus than RA patients without SS. However, the presence of SS did not affect the treatment patterns or autoantibody profiles of RA patients.
The prevalence of SS among Korean RA patients was higher than reported in previous studies conducted in Denmark (3.6%) and Turkey (5.3%) and lower than reported for the USA (10.3%), China (14.5%), and Italy (17.5%).
78151617 Study designs and definitions of SS could have affected previous estimates of SS prevalence among RA patients. The previous studies conducted in Denmark, USA, and Italy were cross-sectional, whereas those in Turkey and China were retrospective. For disease definition, the 2002 AECG criteria were used in the US and Chinese studies, whereas the studies in Denmark, Italy, and Turkey used clinical diagnoses. In Korea, another retrospective study reported that the sicca syndrome was the second most frequent extra-articular manifestation, with prevalence of 7.4% in RA patients.
18 The results of that study were quite similar to ours, but we used a different definition of SS based on objective evidence of glandular dysfunction. In addition, we described the specific serologic profiles and detailed clinical features of SS.
Applying different classification criteria for SS affects the prevalence reported because each set of classification criteria uses distinct objective components, including serologic tests, glandular function tests, and histopathologic confirmation on salivary glands. The most current classification system (the 2016 ACR/EULAR criteria) includes anti-Ro antibody as an independent criterion of the highest weight, so it could be the crucial criterion for diagnosing SS because the summed weights must be ≥ four.
14 In our study, 16 patients did not meet the 2016 ACR/EULAR classification criteria despite having rheumatologist-diagnosed SS (
Supplementary Table 1). All 16 of them underwent the anti-Ro antibody test, and 11 patients had negative results. Among those 11 anti-Ro antibody–negative patients, a minor salivary gland biopsy was not conducted in six patients, and the ocular staining test was not performed in two patients due to patient unwillingness. Thus, the low performance rate of an invasive procedure such a salivary gland biopsy might cause underestimation of SS prevalence according to the 2016 ACR/EULAR classification criteria.
The previous 2002 AECG classification criteria classified SS into primary and secondary, whereas the more recent 2012 ACR and 2016 ACR/EULAR classification criteria focus on primary SS. The 2016 ACR/EULAR classification criteria can also be used to classify secondary SS,
14 and a population-based study supports its performance in defining secondary SS.
19 However, recent criteria might be another hurdle to diagnosing SS among RA patients. The newer classification criteria regard sicca symptoms as the entry criterion for diagnosing SS, and they require RA patients to perform numerous examinations to confirm ocular or salivary gland involvement. In our study, seven of the 60 RA patients who met the 2002 AECG classification criteria did not meet the 2016 ACR/EULAR classification criteria. Among them, four patients did not consent to any testing (ocular staining, Schirmer's test, labial salivary gland biopsy, or unstimulated whole saliva flow). The current criteria might thus delay the diagnosis of SS in RA patients and affect the determination of treatment strategies for RA patients with SS.
As previously noted,
8 RA patients with SS are characterized by a higher frequency of positive serologic tests than found in those without SS. In other words, if a specific autoantibody is positive, the probability that SS will accompany the RA increases; the prevalence of SS increased to 10.5% in RF-positive patients and 17% in patients with ANA titers greater than or equal to 1:80. The anti-Ro antibody has the highest specificity for SS, and 67.6% of the RA patients that were positive for the anti-Ro antibody had SS. Therefore, positive ANA and RF test results, in addition to positivity for the anti-Ro antibody, may be regarded as clues for the diagnosis of SS among RA patients with dryness symptoms. However, the higher rate of positive ANA in this study may have been affected by patients' age, and performance of the ANA test in up to 100% of the patients in the referral hospital. The clinical implications of such autoantibodies should be considered when assessing the various factors that can affect the positivity of autoantibody profiles.
We found no differences in RA manifestations or treatment patterns according to the presence of SS. Because the RA patients with SS were younger than those without SS, they had a lower prevalence of hypertension and diabetes mellitus as comorbidities. In the use of medications, 12.2% of RA patients without SS and 5.6% of RA patients with SS used bDMARDs. However, it is uncertain whether the early use of bDMARDs can delay the development of SS in RA patients. A well-designed prospective study will provide evidence about whether the use of bDMARDs can lower the incidence of SS in RA patients.
This study has several strengths, including our study design. We included all RA patients who visited our rheumatologic clinic within two months to prevent selection bias and confirmation bias. Although the data were collected retrospectively, the diagnostic tests for SS had already been done because our clinic has a well-organized multidisciplinary approach to SS that involves dentists, ophthalmologists, and rheumatologists. Second, we classified SS patients using rheumatologists' diagnoses and the 2002 AECG, 2012 ACR, and 2016 ACR/EULAR classification criteria for SS to show differences in the prevalence of SS among RA patients according to each set of criteria. We thereby clarified potential discrepancies among the criteria and showed the limitations of diagnosing SS in RA patients caused by the insufficient implementation of diagnostic tests in a real-world setting. Third, we provided the prevalence of SS in RA patients according to the presence of sicca symptoms and the presences of RF, ANA, and anti-Ro antibodies. Changes in the prevalence of SS according to the presence of sicca symptoms or autoantibodies implies that they could be potential risk factors for the presence of SS in RA patients.
Our study also has some limitations. First, we might have underestimated the prevalence of SS among RA patients because this was a retrospective observational study from a single tertiary hospital. We could not conduct the invasive studies required to make a final diagnosis of SS unless patients consented to them in that real clinical setting. In the consideration of routine care, we set rheumatologists' diagnostic tests as the gold standard, which resulted in a higher prevalence of SS among RA patients than found in previous studies. Second, we did not collect clinical information about quality of life, fatigue, or sleep quality. In fact, most SS patients suffer from constitutional symptoms, so in further prospective studies, we need to focus not only on dryness but also on patient-reported outcomes (PROs) to better understand RA patients with SS.
In conclusion, we found that the prevalence of SS among RA patients was 8.7%, 7.3%, 6.3%, and 6.8% according to rheumatologists' diagnoses and the 2002 AECG, 2012 ACR, and 2016 ACR/EULAR classification criteria, respectively. Patients with both RA and SS showed a higher rate of positive ANA and anti-Ro antibody tests, and they had a lower prevalence of hypertension and diabetes mellitus than those without SS. However, the clinical characteristics and disease activity of RA did not differ according to the presence of SS. Further prospective observational studies are required to collect PROs from RA patients with SS and to understand how SS affects the clinical course and treatment patterns of RA.









 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download