INTRODUCTION
For patients who have experienced stroke or myocardial infarction (MI), accurate diagnosis and treatment of arrhythmia is crucial for prevention of adverse outcome including additional embolic event or sudden cardiac death (SCD).
1 However, current guidelines do not provide appropriate indication, duration, or specific modality of electrocardiogram (ECG) monitoring for the diagnosis of arrhythmia in these patients.
2
Conventional ECG monitoring devices include multi-lead portable ECG monitoring device, event-detection monitoring device, and implantable ECG monitoring device. However, various drawbacks including several out-patient visits or need of invasive implantation hinder wide use of these devices.
3
Recently, several newer generation ECG monitoring devices with advanced technologies have developed and are under the validation.
4 These new devices have several advantages including light, small and water-proof design, efficient energy use and longer duration of monitoring, wireless data transfer, and no interruption of daily life over conventional Holter recorders.
5 These newer devices can improve quality of life of patients and elongate ECG monitoring duration, diagnostic accuracy and safety should be evaluated.
Zio Patch (iRhythm Technologies, San Francisco, CA, USA) is a single-use, patch type, continuous ECG monitoring device that is attached to the left anterior chest of the patient. This device can continuously monitor the patient's ECG signal for up to 2 weeks.
6 This device received FDA clearance in 2011 and has been prescribed to more than 400,000 patients until recently.
AliveCor KardiaMoblie (AliveCor Inc, Mountain View, CA, USA) is a smartphone-connected ECG monitoring device. The device can measure the single-lead ECG signal of a patient when the 2nd and 3rd fingers of both hands of the patient are placed on the gum-stick sized device. The device can detect symptomatic arrhythmias such as atrial fibrillation (AF) and was cleared by the FDA in 2014.
7
The Apple Watch Series 4 (Apple Inc., Cupertino, CA, USA) is a wrist-band type ECG monitoring device. It records the ECG signal up to 30 seconds when a patient feels prodromal symptoms of arrhythmia and places his opposite finger to the crown of the watch-shaped device.
8
Another patch type ECG monitoring device named ‘ATP100’ (ATsens, Seongnam, Korea) is on the development, its diagnostic capability and safety were not proven from clinical studies yet. So, we sought to compare reliability of this device with conventional multi-lead ECG monitoring device in patients who need ECG monitoring during admission.
METHODS
Study population
We screened consecutive patients who admitted to Seoul National University Bundang Hospital and required continuous ECG monitoring between October 31, 2019 and December 18, 2019. We excluded the patients with 1) implanted pacemaker, cardioverter-defibrillator, or any electrical devices, 2) skin diseases, 3) unstable vital signs, 4) not alert consciousness, 5) inability to give an informed consent. We enrolled 10 patients after obtaining written informed consents.
A new wearable patch-type device
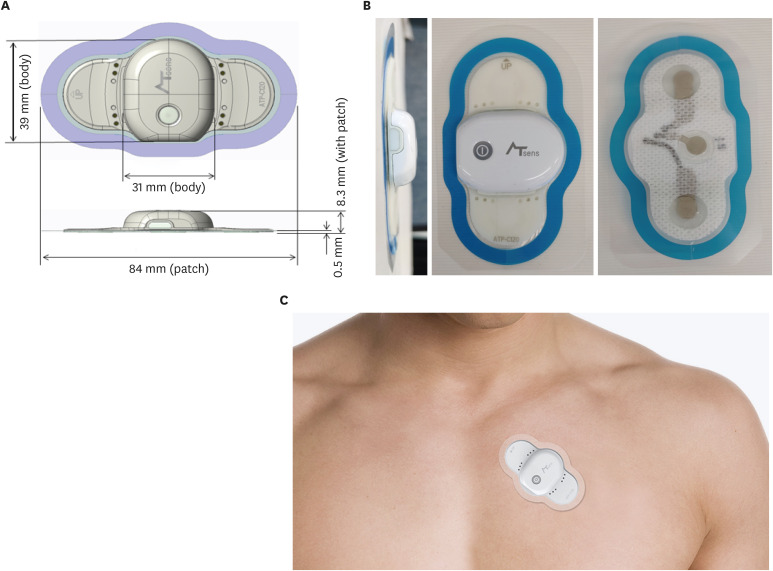
ATP100 is a novel, single-lead ECG monitoring device which can continuously monitor the ECG signal as long as 14 days (11 days if the device is connected to the smartphone via Bluetooth) when attached to skin adjacent to the heart of patients (
Fig. 1). It weighs about 8.3 g, with a size of 84 × 39 × 8.3 mm.
Fig. 1
Information of the ATP100 device. (A) The size of the ATP100 (image courtesy of ATSens, Seongnam, Korea), (B) The photography of the ATP100 (image courtesy of ATSens), (C) The placement of the ATP100 patch (image courtesy of ATSens).

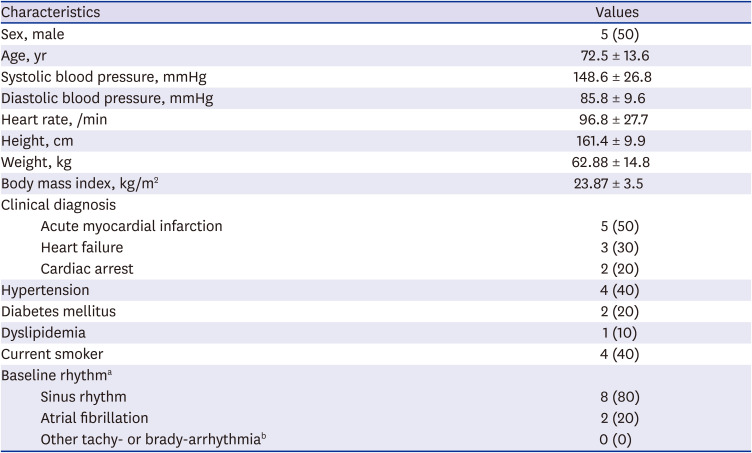
When the device is attached to the patient, several predefined methods were utilized to prevent the occurrence of noise or signal loss. First, the skin was cleansed and disinfected using a 70% ethanol solution. Skin hair was removed if needed. Then the protection film was removed from the patient-side surface of the device. The device is placed at the left 3rd intercostal space 45 degrees tilted toward the inside. Lastly, the device was linked to a smartphone (Galaxy S6; Samsung Electronics, Seoul, Korea) via Bluetooth which recorded continuous ECG signal for 48 hours by a specified application, ATN-C100 (ATsens).
Conventional ECG monitoring
The conventional ECG monitoring device was attached to the patients with the usual in-hospital monitoring protocol using gel conductive ECG electrode pads. To acquire comparator ECG signals, patient parameters such as ECG, respiratory rate, blood-oxygen saturation were measured continuously using IntelliVue MX700 or MP20 (Philips, Amsterdam, Netherlands) in intensive care unit. In cardiology wards equipped with telemonitoring system, IntelliVue TRx4851A (Philips), the continuous ECG was recorded.
During the monitoring period, patients were encouraged to move gently, and stay in the ward where the ECG signal can be detected and monitored. Vigorous movements were not recommended, not only for the signal quality but also due to the patients' medical condition.
ECG signal comparison
All of the comparator continuous ECG recording can be automatically archived and reviewed by IntelliVue Information Center Web™ (Philips). ECG records for 10 seconds after the alarming signal were primarily selected from every patients' record within the predetermined 48 hours. If the alarmed records were over 48 ones in a patient, we selected the records to reflect as many types of arrhythmia as possible at evenly-distributed time interval. If the alarmed records were below 48 ones, we randomly selected the rest records without an alarm at evenly-distributed time interval.
The coincident ECG signals to the comparator ones was archived from the data of smartphones by a program (ATsens) at the recorded time points. The paired 48 signals were analyzed by two cardiologists independently. If there is a disagreement, the sets of signal were sent to a third cardiologist who decided final classification.
Statistical analyses
Data are presented as numbers and frequencies for categorical variables and as mean ± standard deviations for continuous variables. For comparisons between the devices, the χ
2 test (or Fisher exact test when any expected count was < 5 for a 2 × 2 table) was performed for categorical variables. A two-sided probability value of < 0.05 was considered indicative of a statistically significant difference. To evaluate inter-observer reliability and inter-device reliability, Cohen's kappa coefficient was calculated. Statistical analyses were performed using R program version 3.1.0 (The R Foundation for Statistical Computing, Vienna, Austria;
http://www.R-project.org).
Ethics statement
The study protocol was approved by the Institutional Review Board (IRB approval No. E-1903-528-001). The study complied with the principles of the Declaration of Helsinki. Informed consent was submitted by all subjects when they were enrolled.
DISCUSSION
In this preliminary study, we proved that a new portable ECG monitoring device demonstrated comparable results with conventional ECG monitoring devices in detecting arrhythmias. From analyzable ECG data, discrepancy of arrhythmia diagnosis was not observed between the two devices. Noise rate was higher in conventional ECG monitoring device (2.5% vs. 17.3%, P < 0.001) and signal loss was not observed in new adhesive device while there was 9.4% of signal losses in conventional Holter recorder group. The new device was well-tolerated among 48 hours of monitoring period and no adverse event was observed.
In case of conventional monitoring devices, ECG signals were wirelessly transferred to the monitoring system in the cardiology ward. Therefore, there were more chances of signal loss or noise when the patient left the cardiology ward or the ECG signal was interfered with the other radio wave. On the contrary, the ECG signal of ATP100 is primarily stored in the device itself, then transferred to the smartphone. This fail-safe system enabled the newer device to provide less signal loss.
The Multi-lead system of conventional devices is another issue. Because these devices use multiple electrodes that are connected via wires, vigorous movement of the patient can cause lead detachment, and consequently signal loss or noise. On the contrary, ATP100 is a patch type device without any electric wire, which prevented electrode detachment, at least in a short duration of the monitoring period.
Patients with undiagnosed paroxysmal AF can suffer from poorer clinical outcome such as recurrent stroke or systemic embolism, and longer duration ECG monitoring can improve detection rate of AF.
91011 And higher AF burden is also associated with a higher risk of ischemic stroke.
1213
Furthermore, patients who experienced MI bear risk of SCD. Current risk stratification models largely dependent to left ventricular ejection fraction lacks both sensitivity and specificity for prediction of sudden death.
1 Despite the fact that frequent ventricular ectopy or non-sustained VT (NSVT) were associated with post-MI mortality,
14 current guideline does not provide optimal indication or time period of 24-hour ECG monitoring in post-MI patients.
2
Ambulatory ECG can obtain continuous ECG data for more than 1 day without manipulation of the patient and most commonly used diagnostic tool for detection of arrhythmia. And this device can also detect symptom correlation with ECG change. However, its short monitoring duration decreases arrhythmia detection rates and patients may experience multiple tests during their evaluation process. Use of multiple leads, relatively large Holter recorder size, and lack of remote monitoring capability are also main drawbacks. External event recorder provides longer duration of monitoring periods, while its complicated manipulation and inability to detect asymptomatic arrhythmia hinder wide utilization of the device. Implantable loop recorder can address issues aforementioned, but its use is limited by the need of invasive procedure.
3
To mitigate unmet needs of longer duration ECG monitoring without affecting patients’ activities of daily living, various newer ECG monitoring devices have been developed and utilized recently. Some devices are designed to monitor the ECG signal of the subject when he or she feels prodromal symptoms of arrhythmia, such as dizziness, palpitation, or chest discomfort.
AliveCor KardiaMobile (AliveCor Inc, Mountain View, CA, USA) and Apple Watch Series 4 (Apple Inc.) are these sorts of devices. AliveCor KardiaMobile is an electrode-embedded module that is wirelessly connected to the smartphone. When a subject feels chest discomfort, he or she places 2nd and 3rd fingers of both hands to the device simultaneously. Then the device records the 1-lead ECG of patients for about 30 seconds. The recorded signal is transferred to the physician via mail or E-mail. Several studies demonstrate 55%–100% of sensitivity and 84%–99% of specificity in the diagnosis of AF which leads to FDA approval and clinical use of this device.
7151617 Apple Watch Series 4 was also cleared from the FDA in September 2018 and currently available in the USA without prescription. Although this device is easily usable, its clinical efficacy and cost-effectiveness should be evaluated in clinical studies.
8
These kinds of portable self-monitoring devices are easy to use and can correlate symptoms of the patient and rhythm disturbance very well, the accurate diagnosis of asymptomatic arrhythmia is still a challenge. To solve this problem, patch type devices with prolonged ECG monitoring duration are also developed and in the process of validation. ZIO® Patch (iRhythm Technologies, Inc., San Francisco, CA, USA), a single-lead patch type device providing continuous ECG monitoring demonstrated higher rates of arrhythmia detection compared to conventional Holter monitor in several studies.
51819 Its water-proof design and small device size without any electric lead or wire enable prolonged ECG monitoring of patients without bothering their daily activities. Another device named SEEQ® MCT (Medtronic, Inc., Minnesota, USA and Ireland, EU) provides wireless data transfer and real-time analysis.
20
ATP100 is also a patch type continuous ECG monitoring device. Compared to previous patch type devices, ATP100 demonstrates lighter weight and smaller size. Furthermore, relatively short distance between the two electrodes can potentially reduce the amplitude of noise from various origins including skeletal muscle. If the distance between the detector is long, the phase difference of the noise signal should be spread, the noise enlarged. The distance between the detector is 5 cm in ATP100, shorter to competitor device which has a distance of 9 cm.
The device has also adopted several noise filters including high pass filter, low pass filter, and 60 Hz, 50Hz notch filter. With these software-based filters, noise from the movement of body or muscular noise can be reduced significantly. ATP100 demonstrated superior noise control performance compared to the conventional device in this study. Considering that ATP100 can only record a single-lead ECG, the result of the study is noteworthy.
ATP100 also has a water-proof design and capability of real-time ECG analysis, which were not evaluated in this study.
In this study, we demonstrated primary diagnostic capability and safety of newly developed adhesive ECG monitoring device. This device provided similar diagnostic accuracy and superior noise control and image acquisition reliability compared to conventional ECG monitoring system. It could distinguish various arrhythmias, including atrial flutter/fibrillation, ventricular premature beat, sinus pause and Mobitz type I second degree AV block. This result coincided well with other studies conducted with other adhesive ECG monitoring devices before.
Several limitations should be noted. We did not provide the source of the ECG signal to the cardiologists who are conducting the analysis. However, the signals from the comparator device consisted of two lead ECG, while ATP100 device only demonstrated single-lead ECG. Therefore, experienced cardiologists may guess the source of the signal and complete blinding of the device type could not be achieved.
And this is a single-center study based on small number of patients. ECG monitoring duration was limited to 48 hours, and only small fraction of ECG data was included to the analysis. We did not evaluate long-term data acquisition capacity and safety of the device which can operate up to 14 days. No long-term follow-up data was obtained after the study. To validate this device further, a larger scale multi-center study in various clinical situation is needed.