1. Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, et al. Advancing research diagnostic criteria for Alzheimer's disease: the IWG-2 criteria. Lancet Neurol. 2014; 13(6):614–629. PMID:
24849862.

2. McKhann G, Knopman D, Chertkow H, Hyman B, Jack C Jr, Kawas C. The diagnosis of dementia due to Alzheimer's disease: recommendations from the NIAA Association workgroups on diagnostic guidelines for AD. Alzheimers Dement. 2011; 7(3):263–269. PMID:
21514250.
3. Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011; 7(3):270–279. PMID:
21514249.

4. McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011; 7(3):263–269. PMID:
21514250.

5. Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011; 7(3):280–292. PMID:
21514248.

6. Jack CR Jr, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA research framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018; 14(4):535–562. PMID:
29653606.

7. Jack CR Jr, Bennett DA, Blennow K, Carrillo MC, Feldman HH, Frisoni GB, et al. A/T/N: An unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology. 2016; 87(5):539–547. PMID:
27371494.

8. Müller EG, Edwin TH, Stokke C, Navelsaker SS, Babovic A, Bogdanovic N, et al. Amyloid-β PET-Correlation with cerebrospinal fluid biomarkers and prediction of Alzheimer´s disease diagnosis in a memory clinic. PLoS One. 2019; 14(8):e0221365. PMID:
31430334.

9. Jack CR Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, et al. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013; 12(2):207–216. PMID:
23332364.

10. Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, et al. Clinical and biomarker changes in dominantly inherited Alzheimer's disease. N Engl J Med. 2012; 367(9):795–804. PMID:
22784036.

11. Apostolova LG, Hwang KS, Andrawis JP, Green AE, Babakchanian S, Morra JH, et al. 3D PIB and CSF biomarker associations with hippocampal atrophy in ADNI subjects. Neurobiol Aging. 2010; 31(8):1284–1303. PMID:
20538372.

12. Olsson B, Lautner R, Andreasson U, Öhrfelt A, Portelius E, Bjerke M, et al. CSF and blood biomarkers for the diagnosis of Alzheimer's disease: a systematic review and meta-analysis. Lancet Neurol. 2016; 15(7):673–684. PMID:
27068280.

13. Portelius E, Zetterberg H, Skillbäck T, Törnqvist U, Andreasson U, Trojanowski JQ, et al. Cerebrospinal fluid neurogranin: relation to cognition and neurodegeneration in Alzheimer's disease. Brain. 2015; 138(Pt 11):3373–3385. PMID:
26373605.

14. Mattsson N, Insel PS, Palmqvist S, Portelius E, Zetterberg H, Weiner M, et al. Cerebrospinal fluid tau, neurogranin, and neurofilament light in Alzheimer's disease. EMBO Mol Med. 2016; 8(10):1184–1196. PMID:
27534871.

15. Park SA, Chae WS, Kim HJ, Shin HS, Kim S, Im JY, et al. Cerebrospinal fluid biomarkers for the diagnosis of Alzheimer disease in South Korea. Alzheimer Dis Assoc Disord. 2017; 31(1):13–18. PMID:
28030437.

16. McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011; 7(3):263–269. PMID:
21514250.

17. Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999; 56(3):303–308. PMID:
10190820.
19. Park SA, Kang JH, Kang ES, Ki CS, Roh JH, Youn YC, et al. A consensus in Korea regarding a protocol to reduce preanalytical sources of variability in the measurement of the cerebrospinal fluid biomarkers of Alzheimer's disease. J Clin Neurol. 2015; 11(2):132–141. PMID:
25851891.

20. Lee JH, Kim SH, Kim GH, Seo SW, Park HK, Oh SJ, et al. Identification of pure subcortical vascular dementia using 11C-Pittsburgh compound B. Neurology. 2011; 77(1):18–25. PMID:
21593437.

21. Barthel H, Luthardt J, Becker G, Patt M, Hammerstein E, Hartwig K, et al. Individualized quantification of brain β-amyloid burden: results of a proof of mechanism phase 0 florbetaben PET trial in patients with Alzheimer’s disease and healthy controls. Eur J Nucl Med Mol Imaging. 2011; 38(9):1702–1714. PMID:
21547601.

22. Kim SE, Woo S, Kim SW, Chin J, Kim HJ, Lee BI, et al. A nomogram for predicting amyloid PET positivity in amnestic mild cognitive impairment. J Alzheimers Dis. 2018; 66(2):681–691. PMID:
30320571.

23. Kim HJ, Lim TS, Lee SM, Kim TS, Kim Y, An YS, et al. Cerebrospinal fluid levels of β-Amyloid 40 and β-amyloid 42 are proportionately decreased in amyloid positron-emission tomography negative idiopathic normal-pressure hydrocephalus patients. J Clin Neurol. 2019; 15(3):353–359. PMID:
31286708.

24. Jeppsson A, Höltta M, Zetterberg H, Blennow K, Wikkelsø C, Tullberg M. Amyloid mis-metabolism in idiopathic normal pressure hydrocephalus. Fluids Barriers CNS. 2016; 13(1):13. PMID:
27472944.

25. Graff-Radford NR. Alzheimer CSF biomarkers may be misleading in normal-pressure hydrocephalus. Neurology. 2014; 83(17):1573–1575. PMID:
25332445.

26. Blennow K, Hampel H, Weiner M, Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat Rev Neurol. 2010; 6(3):131–144. PMID:
20157306.

27. Buerger K, Ewers M, Pirttilä T, Zinkowski R, Alafuzoff I, Teipel SJ, et al. CSF phosphorylated tau protein correlates with neocortical neurofibrillary pathology in Alzheimer's disease. Brain. 2006; 129(Pt 11):3035–3041. PMID:
17012293.

28. Clark CM, Xie S, Chittams J, Ewbank D, Peskind E, Galasko D, et al. Cerebrospinal fluid tau and beta-amyloid: how well do these biomarkers reflect autopsy-confirmed dementia diagnoses? Arch Neurol. 2003; 60(12):1696–1702. PMID:
14676043.
29. Wahlund LO, Blennow K. Cerebrospinal fluid biomarkers for disease stage and intensity in cognitively impaired patients. Neurosci Lett. 2003; 339(2):99–102. PMID:
12614904.

30. Hansson O, Lehmann S, Otto M, Zetterberg H, Lewczuk P. Advantages and disadvantages of the use of the CSF Amyloid β (Aβ) 42/40 ratio in the diagnosis of Alzheimer's Disease. Alzheimers Res Ther. 2019; 11(1):34. PMID:
31010420.

31. Kim HJ, Lim TS, Lee SM, Kim TS, Kim Y, An YS, et al. Cerebrospinal fluid levels of β-amyloid 40 and β-amyloid 42 are proportionately decreased in amyloid positron-emission tomography negative idiopathic normal-pressure hydrocephalus patients. J Clin Neurol. 2019; 15(3):353–359. PMID:
31286708.

32. Altomare D, de Wilde A, Ossenkoppele R, Pelkmans W, Bouwman F, Groot C, et al. Applying the ATN scheme in a memory clinic population: the ABIDE project. Neurology. 2019; 93(17):e1635–46. PMID:
31597710.
33. Hwang J, Jeong JH, Yoon SJ, Park KW, Kim EJ, Yoon B, et al. Clinical and biomarker characteristics according to clinical spectrum of Alzheimer's disease (AD) in the validation cohort of Korean brain aging study for the early diagnosis and prediction of AD. J Clin Med. 2019; 8(3):E341. PMID:
30862124.

34. Landau SM, Thomas BA, Thurfjell L, Schmidt M, Margolin R, Mintun M, et al. Amyloid PET imaging in Alzheimer's disease: a comparison of three radiotracers. Eur J Nucl Med Mol Imaging. 2014; 41(7):1398–1407. PMID:
24647577.

35. Cho SH, Choe YS, Kim YJ, Kim HJ, Jang H, Kim Y, et al. Head-to-head comparison of 18F-florbetaben and 18F-flutemetamol in the cortical and striatal regions. J Alzheimers Dis. 2020; 76(1):281–290. PMID:
32474468.

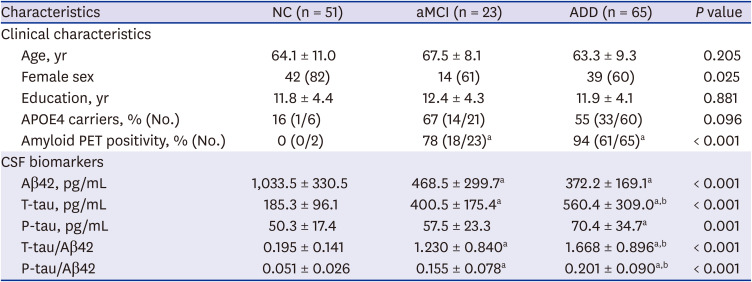
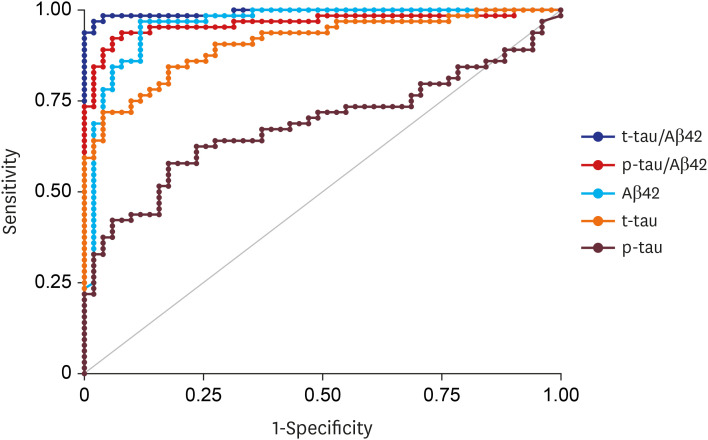
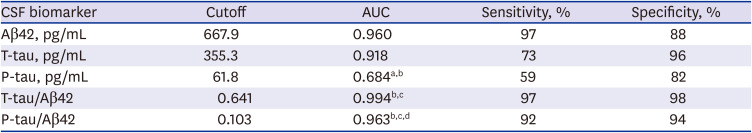
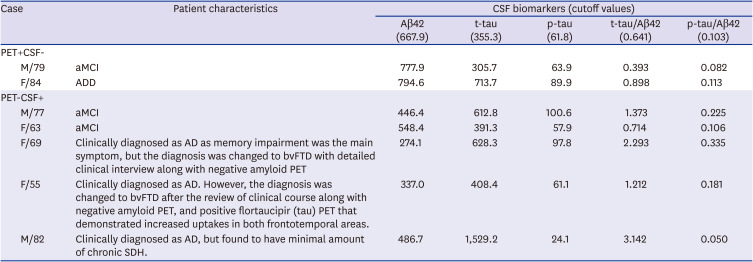




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download