This article has been
cited by other articles in ScienceCentral.
Abstract
Background
Previous studies on the relationship between gastrointestinal (GI) cancer and cholecystectomy remain inconclusive. We aimed to evaluate this relationship, albeit particularly between cholecystectomy and gastric cancer or colorectal cancer (CRC), and the risk factors of cancer among individuals who have undergone cholecystectomy in Korea.
Methods
In total, 4,222 patients who underwent laparoscopic or open cholecystectomy at our institution between January 2006 and December 2013 were included. Patients who underwent cholecystectomy for gallbladder cancer or were undergoing surgery for GI, hepatic, or pancreatobiliary cancers were excluded, as were those who developed stomach cancer or CRC within a year of their cholecystectomy. The included patients were followed until July 20, 2020. The standardized incidence ratio (SIR) was used to calculate the relative risk of GI cancer in cholecystectomy patients.
Results
The median patient age (n = 3,588) at the time of cholecystectomy was 54.0 (range, 19–95) years, and the male-to-female ratio was 1:1.04. The median follow-up period after cholecystectomy was 15.0 (range, 0–146) months. We found a 108% greater risk of CRC (SIR, 2.08; 95% confidence interval [CI], 1.28–3.17) and 154% increased risk of CRC in females (SIR, 2.54; 95% CI, 1.16–4.84). Based on multivariate analysis, an age of > 60 years was a significant risk factor for GI cancer in cholecystectomy patients.
Conclusion
Cholecystectomy may increase risk of CRC, especially in females. Age was considered a risk factor of GI cancers in patients with history of cholecystectomy.
Go to :

Graphical Abstract
Go to :

Keywords: Cholecystectomy, Stomach Cancer, Colorectal Cancer
INTRODUCTION
Owing to the high incidence of gallbladder diseases, cholecystectomy has become increasingly common in abdominal surgery. However, several changes can occur after undergoing this procedure. These include increased bile flow into the duodenum, which leads to increased exposure of the intestine to bile acids, and alterations in the composition of bile salts and metabolic hormone levels. The bile produced by the liver enters directly into the duodenum, and an increased level of bile acids can be carcinogenic. In addition, secondary bile acids can potentially act as toxic endobiotics following a cholecystectomy, and these changes can be correlated to the development of gastrointestinal (GI) cancer.
123
Previous studies on the relationship between GI cancers and cholecystectomy have yielded contrasting results. The risk of developing stomach, colorectal, small intestinal, esophageal, pancreatic, and hepatocellular cancers appear to be higher after cholecystectomy
4567; however, some reports found no relationship between GI cancers and cholecystectomy.
48
The underlying mechanism of cancer development after cholecystectomy differs between organs. In cases of gastric malignancy, an increase in the duodeno-gastric bile reflux has been observed,
9 and the presence of bile in the stomach can cause inflammation,
10 resulting in the development of gastric cancer. Stimulation of the intestinal mucosa by secondary bile acids increase the risk of colorectal cancer (CRC) after cholecystectomy. However, studies assessing the relationship between cholecystectomy and the development of gastric cancer or CRC in Korea are limited.
Therefore, our study aimed to evaluate the relationship between cholecystectomy and gastric cancer or CRC as well as the risk factors of cancers among cholecystectomy patients in Korea.
Go to :

METHODS
A total of 4,222 patients underwent laparoscopic or open cholecystectomy at the institution where this study was conducted between January 2006 and December 2013. Among these patients, those who underwent cholecystectomy for symptomatic cholelithiasis or its complications, gallbladder polyp, or acalculous cholecystitis were included. The exclusion factors were as follows: having undergone cholecystectomy for gallbladder cancer; having undergone concurrent surgery for GI, hepatic, or pancreato-biliary cancers; and having developed stomach cancer or CRC within a year of the cholecystectomy. After excluding 634 patients, 3,588 were finally included in the analysis. Patients were followed until cancer diagnosis, death or end of follow-up (July 20, 2020), whichever occurred first.
Age, sex, height, body weight, lifestyle factors, including alcohol consumption and smoking status, presence of comorbidities such as diabetes mellitus, hypertension, cerebrovascular accidents, cardiovascular diseases, chronic kidney disease, chronic hepatitis B and C, and liver cirrhosis were evaluated at the time of the cholecystectomy. Body mass index (BMI) was calculated using height and weight. Moreover, we assessed whether endoscopic retrograde cholangiopancreatography and/or endoscopic sphincterotomy was performed. The clinical characteristics of the cancers, including location in the stomach and colorectum, stage, treatment, and histological confirmation date were evaluated.
Statistical Package for the Social Sciences version 23 (IBM SPSS, Chicago, IL, USA) was used for the statistical analysis of the data. Categorical variables were presented as numbers and percentages, and continuous variables were presented as mean ± standard deviation or median (range). Student's
t-test and Pearson's χ
2 test were used to assess the difference between variables. Cox regression analysis was performed to determine the correlation of the risk factors to the development of GI cancer in the cholecystectomy patients. The standardized incidence ratio (SIR), which is a ratio of the observed to the expected cases of GI cancer, was used to calculate the relative risk of GI cancers in the cholecystectomy patients. The confidence intervals (CIs) of the SIR were calculated using Poisson distribution.
11 A
P value of < 0.05 was considered statistically significant.
Ethics statement
Owing to the retrospective design of the study, the need for informed consent was waived, and the study was reviewed and approved by the Institutional Review Board of Yeungnam University Hospital (2019-04-059).
Go to :

RESULTS
Participants
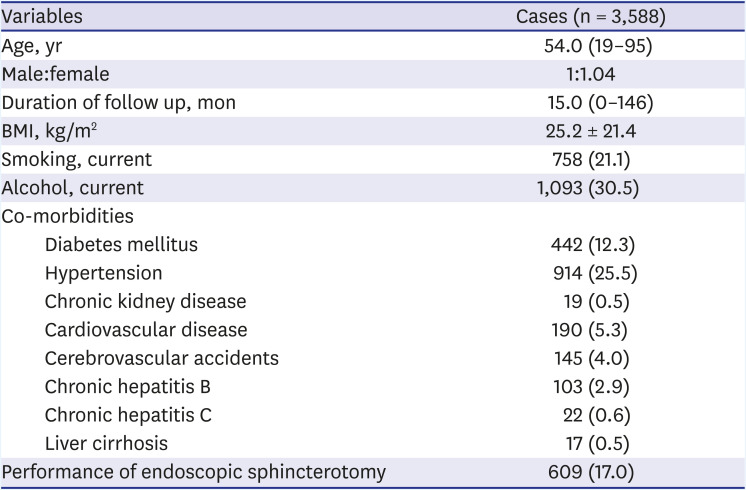
The median age of the patients (n = 3,588) at the time of the cholecystectomy was 54.0 (range: 19–95) years, the male-to-female ratio was 1:1.04, the median follow-up period after the cholecystectomy was 15.0 (range, 0–146) months, and the mean BMI was 25.2 ± 21.4 kg/m
2. In total, 1,093 (30.5%) patients consumed alcohol, and 758 (21.1%) patients were current smokers. Diabetes mellitus was observed in 442 (12.3%) patients and hypertension in 914 (25.5%) patients. Cardiovascular diseases, such as angina and myocardial infarction, were noted in 190 (5.3%) patients, and 145 (4.0%) previously had a cerebrovascular accident. Endoscopic sphincterotomy was performed on 609 (17.0%) patients (
Table 1).
Table 1
Clinical characteristics of patients who underwent cholecystectomy
|
Variables |
Cases (n = 3,588) |
|
Age, yr |
54.0 (19–95) |
|
Male:female |
1:1.04 |
|
Duration of follow up, mon |
15.0 (0–146) |
|
BMI, kg/m2
|
25.2 ± 21.4 |
|
Smoking, current |
758 (21.1) |
|
Alcohol, current |
1,093 (30.5) |
|
Co-morbidities |
|
|
Diabetes mellitus |
442 (12.3) |
|
Hypertension |
914 (25.5) |
|
Chronic kidney disease |
19 (0.5) |
|
Cardiovascular disease |
190 (5.3) |
|
Cerebrovascular accidents |
145 (4.0) |
|
Chronic hepatitis B |
103 (2.9) |
|
Chronic hepatitis C |
22 (0.6) |
|
Liver cirrhosis |
17 (0.5) |
|
Performance of endoscopic sphincterotomy |
609 (17.0) |

SIR of gastric cancer or CRC in cholecystectomy patients
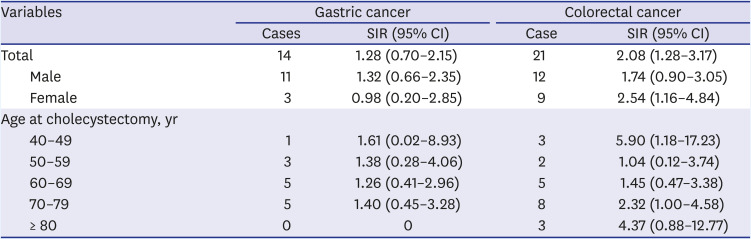
Gastric cancer developed in 14 out of 3,588 patients, while 8.8 patients were expected. The risk of gastric cancer was 28% higher in patients who had undergone cholecystectomy than that in the general population (SIR, 1.28; 95% CI, 0.70–2.15) without significance. Male patients who underwent cholecystectomy had a 32% increased risk of gastric cancer compared with the general population without statistical significance. When compared in terms of age, patients in their 40s had the highest SIR, followed by those in their 70s and then in their 50s.
CRC was diagnosed in 21 cholecystectomy patients and was expected in 10.1 patients. Patients who underwent cholecystectomy had a 108% higher risk of developing CRC than the general population. Moreover, the male and female participants had a 74% and 154% higher risk of CRC, respectively. When compared in terms of age, the SIR of CRC was highest in patients in their 40s, followed by those in their 80s (
Table 2).
Table 2
SIR and 95% CI for gastric and colorectal cancer
|
Variables |
Gastric cancer |
Colorectal cancer |
|
Cases |
SIR (95% CI) |
Case |
SIR (95% CI) |
|
Total |
14 |
1.28 (0.70–2.15) |
21 |
2.08 (1.28–3.17) |
|
Male |
11 |
1.32 (0.66–2.35) |
12 |
1.74 (0.90–3.05) |
|
Female |
3 |
0.98 (0.20–2.85) |
9 |
2.54 (1.16–4.84) |
|
Age at cholecystectomy, yr |
|
|
|
|
|
40–49 |
1 |
1.61 (0.02–8.93) |
3 |
5.90 (1.18–17.23) |
|
50–59 |
3 |
1.38 (0.28–4.06) |
2 |
1.04 (0.12–3.74) |
|
60–69 |
5 |
1.26 (0.41–2.96) |
5 |
1.45 (0.47–3.38) |
|
70–79 |
5 |
1.40 (0.45–3.28) |
8 |
2.32 (1.00–4.58) |
|
≥ 80 |
0 |
0 |
3 |
4.37 (0.88–12.77) |

Clinical characteristics of patients with GI cancer after cholecystectomy
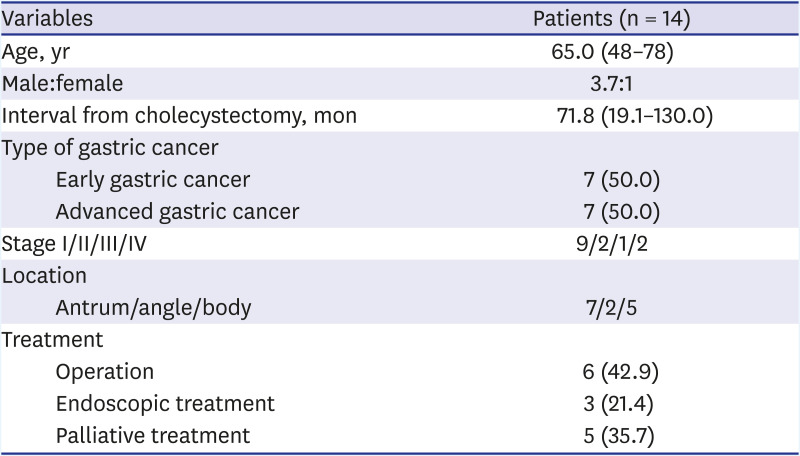
The median duration from the time of the cholecystectomy to the diagnosis of gastric cancer was 71.8 (range, 19.1–130.0) months. The male-to-female ratio was 3.7:1. In total, 7 (50.0%) patients were diagnosed with early-stage gastric cancer and 7 (50.0%) with advanced-stage gastric cancer. Moreover, the numbers of patients with stage I, II, III, and IV gastric cancer were 9, 2, 1, and 2, respectively. Three (21.4%) patients underwent endoscopic resection for curative purposes, and surgical resection was performed on 6 (42.9%) patients (
Table 3).
Table 3
Clinical characteristics of patients diagnosed with gastric cancer after cholecystectomy
|
Variables |
Patients (n = 14) |
|
Age, yr |
65.0 (48–78) |
|
Male:female |
3.7:1 |
|
Interval from cholecystectomy, mon |
71.8 (19.1–130.0) |
|
Type of gastric cancer |
|
|
Early gastric cancer |
7 (50.0) |
|
Advanced gastric cancer |
7 (50.0) |
|
Stage I/II/III/IV |
9/2/1/2 |
|
Location |
|
|
Antrum/angle/body |
7/2/5 |
|
Treatment |
|
|
Operation |
6 (42.9) |
|
Endoscopic treatment |
3 (21.4) |
|
Palliative treatment |
5 (35.7) |

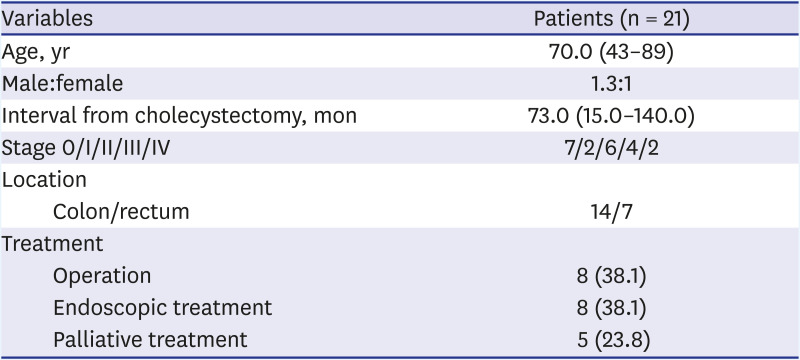
Twenty-one patients were diagnosed with CRC. The male-to-female ratio was 1.3:1, and the median duration from the time of the cholecystectomy to the diagnosis of CRC was 73.0 (range, 15.0–140.0) months. Moreover, the numbers of patients with stage 0, I, II, III, and IV CRC were 7, 2, 6, 4, and 2, respectively. Endoscopic resection was performed on 8 (38.1%) patients, and 8 (38.1%) patients underwent surgical resection (
Table 4).
Table 4
Clinical characteristics of patients diagnosed with colorectal cancer after cholecystectomy
|
Variables |
Patients (n = 21) |
|
Age, yr |
70.0 (43–89) |
|
Male:female |
1.3:1 |
|
Interval from cholecystectomy, mon |
73.0 (15.0–140.0) |
|
Stage 0/I/II/III/IV |
7/2/6/4/2 |
|
Location |
|
|
Colon/rectum |
14/7 |
|
Treatment |
|
|
Operation |
8 (38.1) |
|
Endoscopic treatment |
8 (38.1) |
|
Palliative treatment |
5 (23.8) |

Factors associated with the development of GI cancers after cholecystectomy
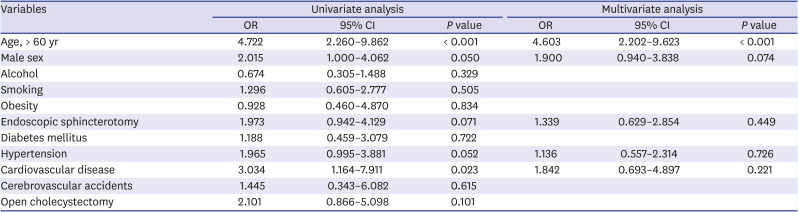
In the univariate analysis, an age of > 60 years (odds ratio [OR], 4.722; 95% CI, 2.260–9.862;
P < 0.001), male sex (OR, 2.015; 95% CI, 1.000–4.062;
P = 0.050), and the presence of cardiovascular disease (OR, 3.034; 95% CI, 1.164–7.911;
P = 0.023) were associated with GI cancers after cholecystectomy. Based on the multivariate analysis, an age of > 60 years (OR, 4.603; 95% CI, 2.202–9.623;
P < 0.001) was a significant risk factor of GI cancer in patients with history of cholecystectomy (
Table 5).
Table 5
Risk factors associated with development of gastric and colorectal cancer after cholecystectomy
|
Variables |
Univariate analysis |
Multivariate analysis |
|
OR |
95% CI |
P value |
OR |
95% CI |
P value |
|
Age, > 60 yr |
4.722 |
2.260–9.862 |
< 0.001 |
4.603 |
2.202–9.623 |
< 0.001 |
|
Male sex |
2.015 |
1.000–4.062 |
0.050 |
1.900 |
0.940–3.838 |
0.074 |
|
Alcohol |
0.674 |
0.305–1.488 |
0.329 |
|
|
|
|
Smoking |
1.296 |
0.605–2.777 |
0.505 |
|
|
|
|
Obesity |
0.928 |
0.460–4.870 |
0.834 |
|
|
|
|
Endoscopic sphincterotomy |
1.973 |
0.942–4.129 |
0.071 |
1.339 |
0.629–2.854 |
0.449 |
|
Diabetes mellitus |
1.188 |
0.459–3.079 |
0.722 |
|
|
|
|
Hypertension |
1.965 |
0.995–3.881 |
0.052 |
1.136 |
0.557–2.314 |
0.726 |
|
Cardiovascular disease |
3.034 |
1.164–7.911 |
0.023 |
1.842 |
0.693–4.897 |
0.221 |
|
Cerebrovascular accidents |
1.445 |
0.343–6.082 |
0.615 |
|
|
|
|
Open cholecystectomy |
2.101 |
0.866–5.098 |
0.101 |
|
|
|

Go to :

DISCUSSION
Previous studies regarding the relationship between GI cancer and cholecystectomy have yielded contradictory results.
45678 Considering that cholecystectomy and the diagnosis of GI cancer are increasingly common, an individual is likely to encounter both in their lifetime. However, the underlying cause of GI cancer after cholecystectomy is challenging to identify. Thus, our study aimed to identify the relationship between cholecystectomy and gastric cancer or CRC among individuals in Korea by excluding patients with other concomitant GI cancer as well as those with gastric cancer and CRC diagnoses obtained within a year of the cholecystectomy.
Cholecystectomy did not increase the risk of gastric cancer in our study, and this result was consistent with that of a previous meta-analysis of six studies.
4 In a previous study of 17 patients who underwent gastric bilirubin monitoring before and after cholecystectomy, cholecystectomy did not increase bile reflux into the stomach.
12 Contrary to our results, several cohort studies have revealed an increased risk of gastric cancer after cholecystectomy.
513
The risk of CRC increased up to 108% after cholecystectomy in our patients compared with that in the general population with statistical significance. The risk of CRC increased in our female patients compared with that in the general population when analyzed by sex. Previous reports regarding the relationship between CRC and cholecystectomy remain inconclusive.
814 A meta-analysis of 10 cohort studies has shown an increased risk of CRC after cholecystectomy,
14 which reflects the results of our study. This finding indicates that screening of CRC in patients with a history of cholecystectomy is required, as is an individualized surveillance program. Early screening for CRC must be conducted on individuals who are in their 40s as they are at the highest risk. However, further studies to evaluate whether colonoscopic surveillance may be beneficial for the early detection of CRC in cholecystectomy patients is warranted.
In our study, a patient age of > 60 years was the only risk factor of gastric cancer or CRC following cholecystectomy. Cerebrovascular disease was only correlated with gastric cancer or CRC after cholecystectomy in the univariate analysis. In previous studies, the risk of CRC was comparatively high after the age of 40,
15 and the risk of gastric cancer also tended to increase with age.
16 Although incidence of gastric cancer is higher in men than in women,
17 sex was not found as a risk factor of gastric cancer after cholecystectomy in this study. The common risk factors of gastric cancer and CRC, which include alcohol consumption, smoking, and obesity, were not considered as risk factors in our study.
Endoscopic sphincterotomy causes damage to the sphincter of Oddi, which can lead to an increased flow of bile into the duodenum. In our study, we evaluated the effect of endoscopic sphincterotomy on the development of gastric cancer or CRC after cholecystectomy. However, an increased risk was not found. With this in mind, damage to the sphincter of Oddi caused by endoscopic sphincterotomy may not increase the risk of gastric cancer and CRC after cholecystectomy. Performance of endoscopic sphincterotomy in patients who have undergone cholecystectomy does not seem to have additional effect on the bile flow into duodenum. If endoscopic sphincterotomy is performed in patients with intact gallbladders, this could potentially affect bile flow into the duodenum.
The current study has several limitations. The retrospective design of this study limits the conclusiveness of the result. Although we excluded patients with gastric cancer or CRC diagnoses obtained within a year after the cholecystectomy, some patients could have had precancerous or early cancer lesions at the time of cholecystectomy. Data from the last endoscopic evaluation before the cholecystectomy could not be obtained. During the follow-up period, lifestyle factors such as smoking and alcohol consumption, weight, and comorbidities might have changed, which could then affect the development of gastric cancer or CRC.
Despite these limitations, our study attempted to identify the relationship between cholecystectomy and gastric cancer and CRC, both of which are common diseases in Korea. Moreover, SIR was used to compare the risk of GI cancers between patients who underwent cholecystectomy and the general population. In addition, a relatively large number of patients who underwent cholecystectomy at a single institution was analyzed. The accessibility of endoscopy is high in Korea, which lent itself well to the evaluation of the relationship between gastric cancer, CRC, and cholecystectomy in this study.
In conclusion, cholecystectomy might increase the risk of CRC, especially in females. Age was considered as a risk factor of GI cancers in patients with history of cholecystectomy.
Go to :










 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download