1. Berger A, Rahmi G, Perrod G, Pioche M, Canard JM, Cesbron-Métivier E, et al. Long-term follow-up after endoscopic resection for superficial esophageal squamous cell carcinoma: a multicenter Western study. Endoscopy. 2019; 51(4):298–306. PMID:
30261535.

2. Min YW, Lee H, Song BG, Min BH, Kim HK, Choi YS, et al. Comparison of endoscopic submucosal dissection and surgery for superficial esophageal squamous cell carcinoma: a propensity score-matched analysis. Gastrointest Endosc. 2018; 88(4):624–633. PMID:
29750981.

3. Furue Y, Katada C, Tanabe S, Ishido K, Kondo Y, Kubota Y, et al. Effectiveness and safety of endoscopic aspiration mucosectomy and endoscopic submucosal dissection in patients with superficial esophageal squamous-cell carcinoma. Surg Endosc. 2019; 33(5):1433–1440. PMID:
30187201.

4. Song BG, Min YW, Lee JH, Lee H, Min BH, Rhee PL, et al. Efficacy and safety of endoscopic submucosal dissection in elderly patients with esophageal squamous cell carcinoma. Surg Endosc. 2017; 31(10):3905–3911. PMID:
28342128.

5. Choi JY, Park YS, Jung HY, Ahn JY, Kim MY, Lee JH, et al. Feasibility of endoscopic resection in superficial esophageal squamous carcinoma. Gastrointest Endosc. 2011; 73(5):881–889. 889.e1–882. PMID:
21392755.

6. Puli SR, Reddy JB, Bechtold ML, Antillon D, Ibdah JA, Antillon MR. Staging accuracy of esophageal cancer by endoscopic ultrasound: a meta-analysis and systematic review. World J Gastroenterol. 2008; 14(10):1479–1490. PMID:
18330935.

7. Thosani N, Singh H, Kapadia A, Ochi N, Lee JH, Ajani J, et al. Diagnostic accuracy of EUS in differentiating mucosal versus submucosal invasion of superficial esophageal cancers: a systematic review and meta-analysis. Gastrointest Endosc. 2012; 75(2):242–253. PMID:
22115605.

8. Lightdale CJ, Kulkarni KG. Role of endoscopic ultrasonography in the staging and follow-up of esophageal cancer. J Clin Oncol. 2005; 23(20):4483–4489. PMID:
16002838.

9. Oyama T, Inoue H, Arima M, Momma K, Omori T, Ishihara R, et al. Prediction of the invasion depth of superficial squamous cell carcinoma based on microvessel morphology: magnifying endoscopic classification of the Japan Esophageal Society. Esophagus. 2017; 14(2):105–112. PMID:
28386209.

10. Qu J, Zhang H, Wang Z, Zhang F, Liu H, Ding Z, et al. Comparison between free-breathing radial VIBE on 3-T MRI and endoscopic ultrasound for preoperative T staging of resectable oesophageal cancer, with histopathological correlation. Eur Radiol. 2018; 28(2):780–787. PMID:
28799124.

11. Young PE, Gentry AB, Acosta RD, Greenwald BD, Riddle M. Endoscopic ultrasound does not accurately stage early adenocarcinoma or high-grade dysplasia of the esophagus. Clin Gastroenterol Hepatol. 2010; 8(12):1037–1041. PMID:
20831900.

12. Goda K, Tajiri H, Ikegami M, Yoshida Y, Yoshimura N, Kato M, et al. Magnifying endoscopy with narrow band imaging for predicting the invasion depth of superficial esophageal squamous cell carcinoma. Dis Esophagus. 2009; 22(5):453–460. PMID:
19222533.

13. May A, Günter E, Roth F, Gossner L, Stolte M, Vieth M, et al. Accuracy of staging in early oesophageal cancer using high resolution endoscopy and high resolution endosonography: a comparative, prospective, and blinded trial. Gut. 2004; 53(5):634–640. PMID:
15082579.

14. Endoscopic Classification Review Group. Update on the paris classification of superficial neoplastic lesions in the digestive tract. Endoscopy. 2005; 37(6):570–578. PMID:
15933932.
15. Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011; 14(2):101–112. PMID:
21573743.
16. Brierley JD, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours. 8th ed. Chichester: John Wiley & Sons;2017.
17. Lokuhetty D, White VA, Watanabe R, Cree IA. World Health Organization. Digestive System Tumours. 5th ed. Lyon: International Agency for Research on Cancer;2019.
18. Yoshida T, Inoue H, Usui S, Satodate H, Fukami N, Kudo SE. Narrow-band imaging system with magnifying endoscopy for superficial esophageal lesions. Gastrointest Endosc. 2004; 59(2):288–295. PMID:
14745410.

19. Lee MW, Kim GH, I H, Park DY, Baek DH, Lee BE, et al. Predicting the invasion depth of esophageal squamous cell carcinoma: comparison of endoscopic ultrasonography and magnifying endoscopy. Scand J Gastroenterol. 2014; 49(7):853–861. PMID:
24957951.

20. Nakagawa K, Ishihara R, Aoyama K, Ohmori M, Nakahira H, Matsuura N, et al. Classification for invasion depth of esophageal squamous cell carcinoma using a deep neural network compared with experienced endoscopists. Gastrointest Endosc. 2019; 90(3):407–414. PMID:
31077698.

21. Participants in the Paris Workshop. The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003; 58(6):Suppl. S3–S43. PMID:
14652541.
22. Qin X, He S, Zhang Y, Xue L, Lu N, Wang G. Diagnosis and staging of superficial esophageal precursor based on pre-endoscopic resection system comparable to endoscopic resection. BMC Cancer. 2014; 14(1):774. PMID:
25330811.

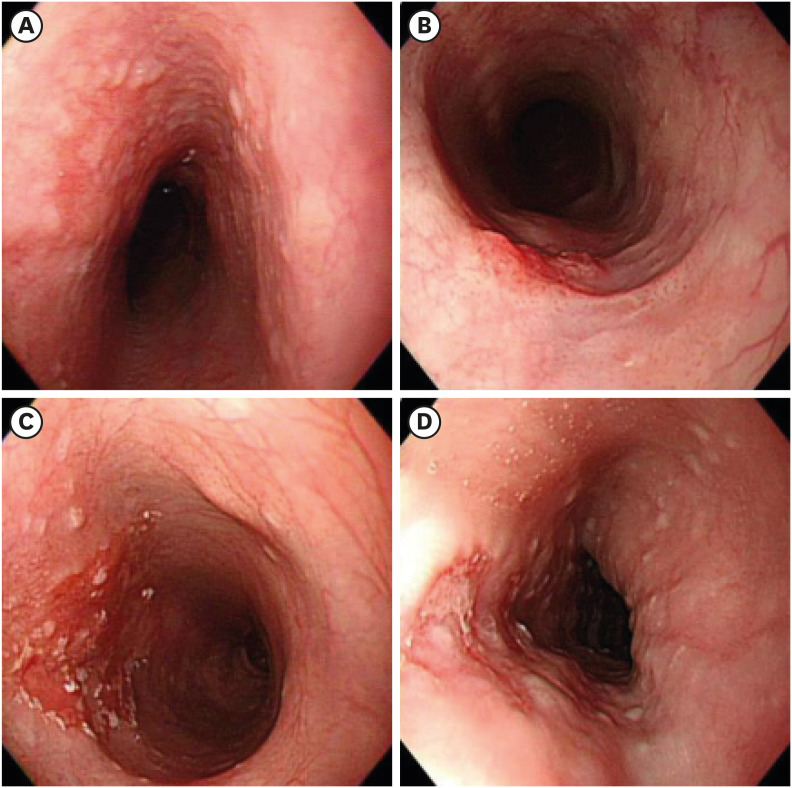
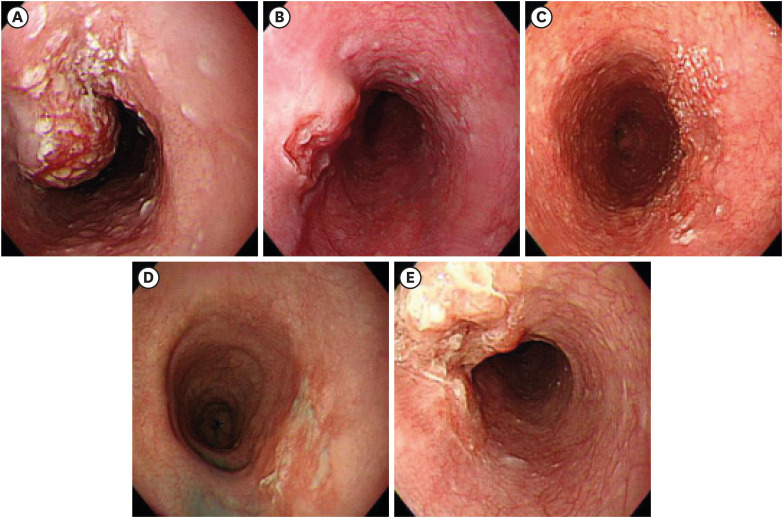
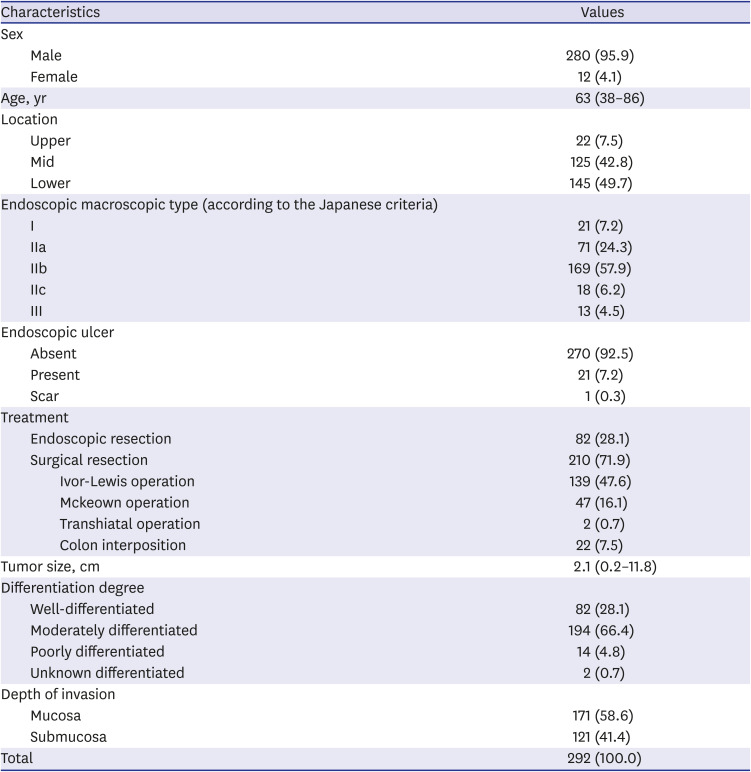
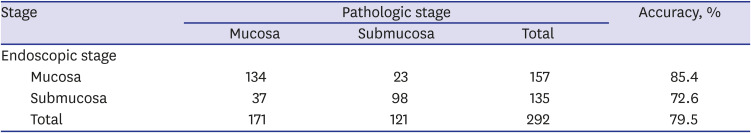




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download