METHODS
We reviewed the cases confirmed by the Korea Advisory Committee on Vaccine Injury Compensation (KACVIC) under the KCDC with vaccine anaphylaxis from 2001 to 2016 and used the National Vaccine and Injury Investigation Team's reports.
Data collected included demographic information and medical history from medical records. We investigated whether there were any adverse reactions from recipients who had been vaccinated with the same lot number. We evaluated vaccine management states, such as vaccine refrigerator operation, temperature measurement methods, vaccination records, national test reports, biological product shipping certificates, and performance certificates.
Ethics statement
Institutional Review Board of Incheon Medical Center reviewed the study and exempted it from deliberation (115288-201805-HR-028-01).
RESULTS
During the period, there were 13 cases of vaccine-related anaphylaxis and no deaths (
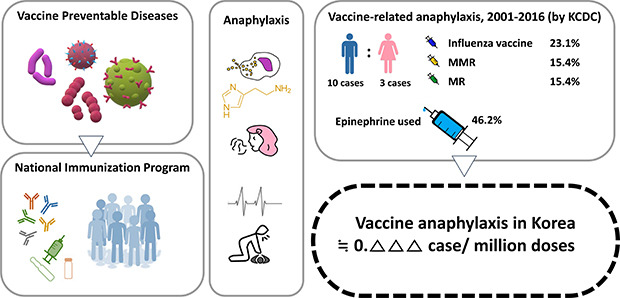
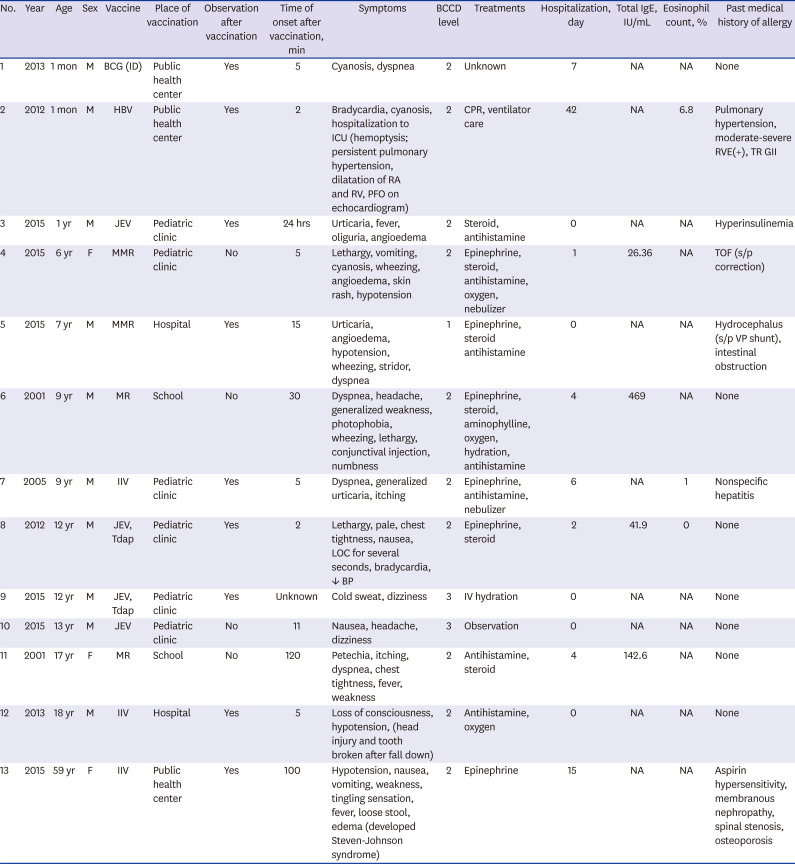
Table 1). The median age was 9 years (range, 1 month to 59 years), and males represented 10 (76.8%) cases. They were all fully recovered. The incidence of anaphylaxis per million doses was 0.090 in 2005, 0.079 in 2012, 0.071 in 2013, 0.188 in 2015, and 0.036 in 2016. The incidence for 2001 was not calculated because the denominator and dose for each vaccine were unknown.
Table 1
Clinical findings of 13 vaccine-related anaphylaxis cases, confirmed by KCDC during 2001–2016
|
No. |
Year |
Age |
Sex |
Vaccine |
Place of vaccination |
Observation after vaccination |
Time of onset after vaccination, min |
Symptoms |
BCCD level |
Treatments |
Hospitalization, day |
Total IgE, IU/mL |
Eosinophil count, % |
Past medical history of allergy |
|
1 |
2013 |
1 mon |
M |
BCG (ID) |
Public health center |
Yes |
5 |
Cyanosis, dyspnea |
2 |
Unknown |
7 |
NA |
NA |
None |
|
2 |
2012 |
1 mon |
M |
HBV |
Public health center |
Yes |
2 |
Bradycardia, cyanosis, hospitalization to ICU (hemoptysis; persistent pulmonary hypertension, dilatation of RA and RV, PFO on echocardiogram) |
2 |
CPR, ventilator care |
42 |
NA |
6.8 |
Pulmonary hypertension, moderate-severe RVE(+), TR GII |
|
3 |
2015 |
1 yr |
M |
JEV |
Pediatric clinic |
Yes |
24 hrs |
Urticaria, fever, oliguria, angioedema |
2 |
Steroid, antihistamine |
0 |
NA |
NA |
Hyperinsulinemia |
|
4 |
2015 |
6 yr |
F |
MMR |
Pediatric clinic |
No |
5 |
Lethargy, vomiting, cyanosis, wheezing, angioedema, skin rash, hypotension |
2 |
Epinephrine, steroid, antihistamine, oxygen, nebulizer |
1 |
26.36 |
NA |
TOF (s/p correction) |
|
5 |
2015 |
7 yr |
M |
MMR |
Hospital |
Yes |
15 |
Urticaria, angioedema, hypotension, wheezing, stridor, dyspnea |
1 |
Epinephrine, steroid antihistamine |
0 |
NA |
NA |
Hydrocephalus (s/p VP shunt), intestinal obstruction |
|
6 |
2001 |
9 yr |
M |
MR |
School |
No |
30 |
Dyspnea, headache, generalized weakness, photophobia, wheezing, lethargy, conjunctival injection, numbness |
2 |
Epinephrine, steroid, aminophylline, oxygen, hydration, antihistamine |
4 |
469 |
NA |
None |
|
7 |
2005 |
9 yr |
M |
IIV |
Pediatric clinic |
Yes |
5 |
Dyspnea, generalized urticaria, itching |
2 |
Epinephrine, antihistamine, nebulizer |
6 |
NA |
1 |
Nonspecific hepatitis |
|
8 |
2012 |
12 yr |
M |
JEV, Tdap |
Pediatric clinic |
Yes |
2 |
Lethargy, pale, chest tightness, nausea, LOC for several seconds, bradycardia, ↓ BP |
2 |
Epinephrine, steroid |
2 |
41.9 |
0 |
None |
|
9 |
2015 |
12 yr |
M |
JEV, Tdap |
Pediatric clinic |
Yes |
Unknown |
Cold sweat, dizziness |
3 |
IV hydration |
0 |
NA |
NA |
None |
|
10 |
2015 |
13 yr |
M |
JEV |
Pediatric clinic |
No |
11 |
Nausea, headache, dizziness |
3 |
Observation |
0 |
NA |
NA |
None |
|
11 |
2001 |
17 yr |
F |
MR |
School |
No |
120 |
Petechia, itching, dyspnea, chest tightness, fever, weakness |
2 |
Antihistamine, steroid |
4 |
142.6 |
NA |
None |
|
12 |
2013 |
18 yr |
M |
IIV |
Hospital |
Yes |
5 |
Loss of consciousness, hypotension, (head injury and tooth broken after fall down) |
2 |
Antihistamine, oxygen |
0 |
NA |
NA |
None |
|
13 |
2015 |
59 yr |
F |
IIV |
Public health center |
Yes |
100 |
Hypotension, nausea, vomiting, weakness, tingling sensation, fever, loose stool, edema (developed Steven-Johnson syndrome) |
2 |
Epinephrine |
15 |
NA |
NA |
Aspirin hypersensitivity, membranous nephropathy, spinal stenosis, osteoporosis |

Most immunization places were in pediatric outpatient clinics (8 cases), followed by public health centers (3 cases) and schools (2 cases). Anaphylaxis that occurred after vaccination was most frequent within 30 minutes (76.9%), followed by 30 minutes to 2 hours (15.4%), and after 2 hours (7.7%). The median time of symptom onset was 11 minutes. They all had no history of anaphylaxis or allergic reaction.
Reported vaccines were influenza (3/13, 23.1%), measles-mumps-rubella vaccine (MMR; 2/13, 15.4%), measles-rubella vaccine (MR; 2/13, 15.4%), inactivated Japanese encephalitis vaccine (JEV) alone (2/13, 15.4%), JEV and tetanus-diphtheria-acellular pertussis vaccine (Tdap) together (2/13, 15.4%), Bacille Calmette-Guérin vaccine (BCG) intradermal type (1 case, 7.7%) and hepatitis B vaccine (HBV; 1 case, 7.7%). Two cases were MR vaccinated at school by the ‘Catch-up Campaign’ program when there was a measles epidemic in Korea in 2000–2001. In 2015, there were 2 cases of JEV single injection, 1 JEV and Tdap simultaneous injection, and 2 MMR. These were identified as different lot numbers.
Most of the patients manifested cardiovascular symptoms (84.6%). Respiratory (61.5%), dermatologic (46.2%) and gastrointestinal system (38.5%) symptoms were also prevalent. The results of the BCCD level according to the Adverse Event Following Immunization (AEFI) reporting checklist were 10/13 (76.9%) for level 2, 2/13 (15.4%) for level 3, 1/13 was level 1 (7.7%).
Six cases (46.2%) were treated with epinephrine, six cases (46.2%) were treated with corticosteroid, and seven cases (53.8%) were treated with anti-histamine medication. Cardiopulmonary resuscitation was performed on one patient who was one month of age. He was diagnosed with congenital heart disease.
The rate of hospitalization was 61.3% (8/13), and the median duration was 10 days (1–42 days). Five cases (38.5%) had improved without hospitalization and counsel regarding future vaccinations. Nine people were monitored for sufficient time after vaccination at the immunization place. Four cases had not been observed for enough time after the immunization and one of them fell from the anaphylactic shock and sustained a head and tooth injury.
DISCUSSION
By implementing NIP, the world has controlled VPD, achieving herd immunity as well as immunization of those who are vaccinated.
8 In Korea, an advisory committee on immunization was formed in 1992 to compensate victims of vaccination.
3 In 1994, there had been four cases of death after the JEV inoculation, then the AEFI Surveillance System and the National Vaccine Injury Composition Program were subsequently established.
3 Immunization rates in Korea averaged 97.2% in 2018, higher than in the United States (86.9%), Australia (94.3%), and the United Kingdom (93.9%).
9
In the US, vaccine-induced anaphylaxis was rare at 1.31 per million doses across all ages during 2009–2011.
10 Also in Germany, except for the influenza A virus subtype (H1N1) pandemic influenza vaccination, it was estimated at 6.8 cases per year.
11 In the previous study 2009–2013 in Korea, vaccines had been reported being responsible for 1.0 percent of the cases.
12 However, this was limited in identifying the frequency of vaccine anaphylaxis because it was a report by a small number of medical institutions and the result was a code analysis using the International Classification of Diseases, 10th Revision (ICD-10), but not a BCCD based analysis. In reporting to AEFI, BCCD helps to determine a causal relationship between anaphylaxis and vaccines and is used as a standard checklist in analyzing the surveillance data.
1 An important finding in our study is that it included anaphylactic cases in which KCDC determined there was a definite causal relationship with a vaccine by using BCCD.
As part of NIP in Korea, the KCDC has been operating the national immunization safety management system; securing high-quality vaccines, monitoring adverse reactions, conducting epidemiological investigations of serious adverse reactions, and managing a vaccine injury compensation system.
2
Vaccine components include immunizing antigens such as toxoids and viral proteins, egg or yeast as culture media, antibiotics, thimerosal preservatives as additives, gelatin or albumin stabilizers, and aluminum salts. These are all potential allergens, and even latex on the vaccine lid can trigger an allergic reaction during the handling process.
131415 In the production of recombinant HBV,
Saccharomyces cerevisiae is used during the cell culture process. Among hosts with yeast allergy, anaphylaxis may rarely occur.
16
In the US, anaphylaxis by inactivated trivalent influenza vaccine and monovalent were 1.35 and 1.83 per million doses, respectively, and in Germany, anaphylaxis was most common after the immunization of the pandemic H1N1 influenza vaccine.
1011 One study found that seven out of 10 cases of vaccine anaphylaxis were caused by influenza vaccines in Korea.
12
The influenza vaccine was the most common cause of adverse reactions in 2008-2012 in Korea.
13 In particular, 12 million monovalent influenza vaccines had been given during pandemic H1N1 in 2009, of which 23 had been suspected of anaphylaxis, which was 0.18 cases per 100,000 population.
513 Influenza vaccines were also the most common cause of anaphylaxis (23.1%) in our study, which confirmed the causal relationship with vaccines.
A 59-year-old woman with a history of aspirin-exacerbated respiratory disease developed Steven Johnson syndrome (SJS) five days after influenza vaccination. There is no convincing data the influenza vaccine caused SJS. The possibility of other causes could not be ruled out, such as drugs during hospitalization or taken before vaccination. But in a case report in Japan in 2016, a 75-year-old man developed the SJS two days after the flu vaccine.
17
It should be remembered that some of the risk factors for severe anaphylaxis include old age, comorbid medical conditions, and asthma.
10181920
Epinephrine is a drug that is used in the treatment of severe anaphylaxis, but health care workers hesitate to use it. In our findings, epinephrine had been administered in six cases (46.2%). In Australia, 72% of anaphylaxis patients were treated with adrenaline, whereas in Korea, 36.9% of adults and 24% of children were administered epinephrine.
2122
There was one person who collapsed after immunization, causing tooth and head injury. Usually children should be observed for 20 to 30 minutes after vaccination, but children at high-risk of anaphylaxis should be observed for 60 minutes and restricted from strenuous exercise for one day.
23
A limitation of our research is that detailed medical information and circumstances were not available in some cases. As the frequency of vaccine-related anaphylaxis itself was very small, the risk factors for anaphylaxis could not be determined. The incidence from these results was calculated only in cases where the vaccine was identified as vaccine anaphylaxis and rewarded. Therefore, vaccine-related anaphylaxis cases may be under-reported and may be higher than the results reported in this study.
The AEFI surveillance system was introduced in 1994, but the computerization of the Electronic Document Interchange (EDI) system began in 2000.
4 The frequency of some years could not be calculated because the total number of vaccination corresponding to the denominator was unknown.
The prospective analysis and continuous monitoring of AEFI are required.
We suggest that to prevent severe vaccine anaphylaxis, 1) a careful history of anaphylaxis or allergy should be obtained, 2) there should be adequate observation time after immunization, and 3) differential diagnosis of anaphylaxis, and preparation of epinephrine for an emergency are needed.
In conclusion, the incidence of vaccine-related anaphylaxis was very low in Korea. The anaphylaxis cases had been classified according to BCCD levels, and these results can be used as national representative data for vaccine-anaphylaxis in Korea.
Vaccination of the NIP is very safe and has contributed to disease prevention by increasing the vaccination coverage rate. Nevertheless, physicians should always be aware of the potential for fatal adverse reactions after vaccination, be prepared for emergencies, and actively treat anaphylaxis.
The risks and precautions should be evaluated before immunization to prevent vaccine-related anaphylaxis. Whenever new vaccines are introduced, vaccine-related side effects and the frequency of anaphylaxis should be always monitored.