INTRODUCTION
At the end of 2019, a novel coronavirus was identified as the cause of a cluster of pneumonia cases in Wuhan, China.
1 It rapidly spread, resulting in an epidemic throughout China, followed by an explosion of cases in most countries worldwide. The World Health Organization (WHO) characterized coronavirus disease 2019 (COVID-19) as a pandemic.
2 As of July 12, 2020, over 12 million confirmed cases including over 0.5 million deaths had been documented globally.
3
The understanding of COVID-19 is continuously evolving. However, the known information focuses on inpatients with severe conditions or residents in specific environments, whereas information on asymptomatic patients or those with mild symptoms and residents in the community is limited.
14567
Ulsan is Korea's seventh-largest metropolitan city with over 1.1 million inhabitants. On February 22, 2020, the first confirmed case of COVID-19 was reported in Ulsan, and by April 26, there were 40 cases. Most COVID-19 patients in Ulsan had mild symptoms or were asymptomatic and were detected by screening individuals suspected of being exposed to severe acute respiratory syndrome coronavirus-2 (SARS-CoV2). For public health purposes, all COVID-19 cases confirmed in this city were admitted to a designated hospital, i.e., Ulsan University Hospital (UUH; Ulsan, Korea), regardless of the disease severity. Therefore, the evaluation of COVID-19 patients at UUH will likely provide information about mild or asymptomatic COVID-19 patients in the community.
We analyzed the clinical characteristics of all consecutively hospitalized COVID-19 patients at UUH.
METHODS
Study design
This retrospective case series was conducted at UUH, the largest teaching hospital in Ulsan.
Study patients
All patients were Korean residents of Ulsan and were diagnosed with COVID-19 by real-time reverse transcription-polymerase chain reaction (RT-PCR) for SARS-CoV-2. Among the 40 patients, 38 were confirmed at 10 screening centers in Ulsan and 2 at 2 screening centers in other cities and referred by the respective centers. The screening test for SARS-CoV2 was obligatory for families or those in close contacts with confirmed COVID-19 patients in Korea. Patients were admitted to UUH between February 22, 2020, and March 26, 2020. Clinical outcomes were monitored until May 19, 2020, the date when the last patient was discharged.
Data sources and variables
Demographic, clinical, and laboratory variables were collected from the clinical data warehouse platform in conjunction with the electronic medical records at the UUH Information of Clinical Ecosystem, and the authors manually reviewed these medical records. Additionally, we referred to epidemiological and clinical data identified by a preventive medicine specialist who interviewed the patients at the UUH and another source of epidemiological data for Ulsan. Transfers from UUH to a local hospital were merged and considered a single visit. Regarding patients who were readmitted during the study period, data from the first admission were considered. Data were collected on demographic characteristics (age, sex, occupation, education, and religion), epidemiological information, triage vitals (blood pressure, heart rate, temperature, respiratory rate, and peripheral oxygen saturation), comorbidities (hypertension, diabetes, dyslipidemia, cardiovascular disease, cancer, and hepatitis B infection), history of clinical signs and symptoms, laboratory and radiologic findings, medications (antiviral therapy and antimalarial therapy), treatments (continuous renal replacement therapy and invasive mechanical ventilation), outcomes (discharge, readmission, and mortality), and main durations (incubation period, symptom onset to hospital admission, viral shedding, and hospital stay). We calculated the Modified Early Warning Score (MEWS)
89 and CURB-65
101112 pneumonia severity score. MEWS is based on systolic blood pressure, heart rate, respiratory rate, temperature, and consciousness, whereas CURB-65 is based on confusion, urea levels, respiratory rate, blood pressure, and ≥ 65 years. Fever was defined as a temperature of ≥ 37.5°C or subjective fever before admission and a temperature of ≥ 37.5°C in axillary measurements obtained after admission. Laboratory and radiologic findings were considered from the first test results available, typically within 24 hours of admission. When all symptoms and signs, radiologic findings, and laboratory findings of COVID-19 subsided and two consecutive negative results for SARS-CoV-2 infection were reported, the patient was considered recovered from COVID-19 and was discharged. If a patient had persistent positive SARS-CoV-2 test results and the other parameters were normalized, the patient was referred to a local hospital specified for Ulsan and hospitalized until negative results were obtained in two consecutive SARS-CoV-2 tests.
Real-time RT-PCR for SARS-CoV-2
During hospitalization, respiratory tract specimens including sputum and nasopharyngeal or oropharyngeal swab samples were collected to detect SARS-CoV-2 on different dates. After viral RNA extraction with the NX-48 viral nucleic acid extraction kit (Genolution, Seoul, Korea) in conjunction with Nextractor NX-48 (Genolution), real-time RT-PCR was performed using the PowerChek™ 2019-nCoV Real-Time PCR Kit (KogeneBiotech, Seoul, Korea) with the Bio-Rad CFX96 Deep Well Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA). This assay targets two genes (E: for
Sarbecovirus screening and RdRp for confirmation of SARS-CoV-2), as suggested by the Korea Centers for Disease Control and Prevention and WHO.
13
Symptoms and signs
We created a list of symptoms from previous COVID-19 studies.
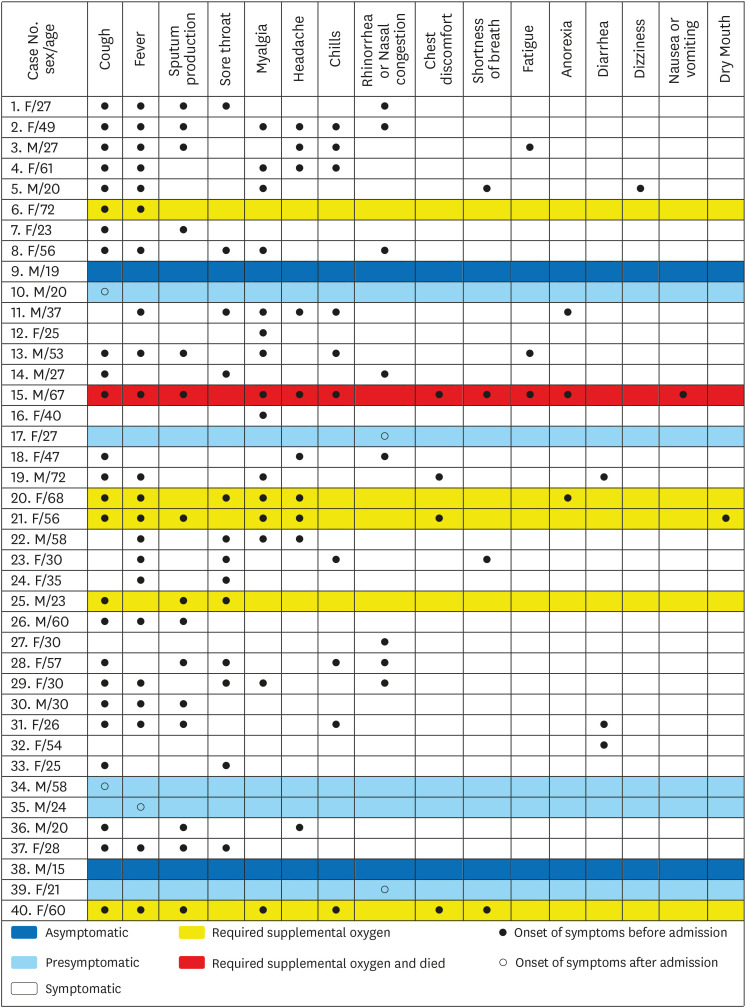
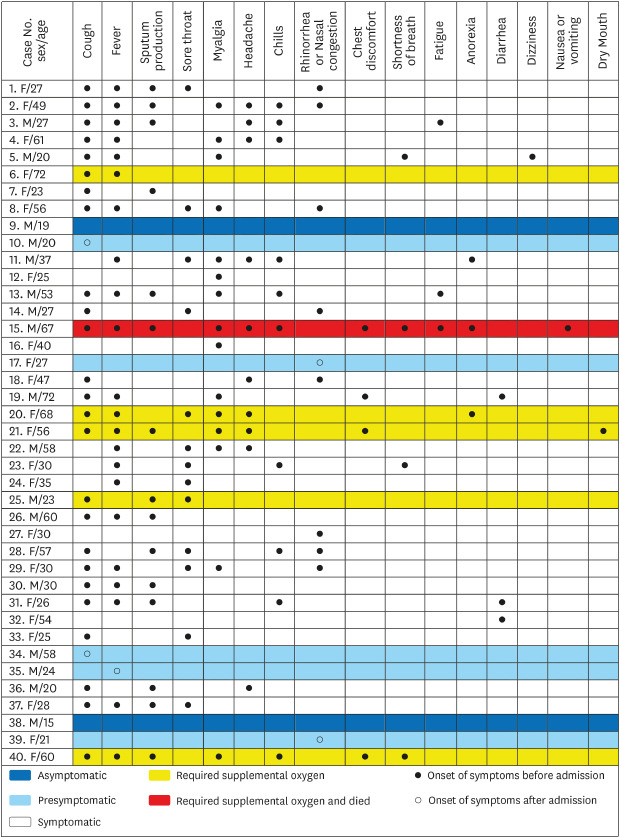
4561415 Patients were classified as symptomatic if they had fever, cough, sputum production, sore throat, myalgia, headache, chills, rhinorrhea or nasal congestion, chest discomfort, shortness of breath, fatigue, anorexia, diarrhea, nausea or vomiting, or dry mouth for the duration from suspected exposure to SARS-CoV-2 to hospital admission. Presymptomatic patients were defined as those who had no symptoms before admission but developed symptoms during admission. Asymptomatic patients were those who had no symptoms at any time for the duration from suspected exposure to SARS-CoV-2 to hospital discharge.
Main durations
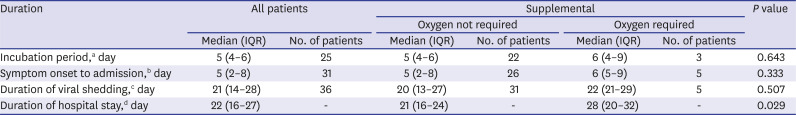
The incubation period was estimated based on the date of estimated exposure to SARS-CoV-2 to the date of symptom onset. Patients who were asymptomatic or symptomatic without the exact dates of exposure to SARS-CoV-2 or symptom onset known were excluded from this analysis. The duration from symptom onset to hospital admission was analyzed in symptomatic patients. Patients who were asymptomatic, presymptomatic, or symptomatic without the exact date of symptom onset known were excluded. To estimate the duration of viral shedding, we defined the date of nucleic acid conversion as the median of the date of the last positive real-time RT-PCR test result and the date of the first negative real-time RT-PCR test because real-time RT-PCR was not performed daily. The test intervals ranged from 1 to 7 days according to patient conditions. Therefore, the duration of viral shedding was calculated by subtracting the date of symptom onset from the median of the date of the last positive real-time RT-PCR test result and the date of the first negative real-time RT-PCR test result. Patients who were asymptomatic or symptomatic without the exact date of symptom onset known were also excluded. Duration of hospital stay was calculated by subtracting the date of admission from the date of discharge. Concerning patients who were readmitted, the durations of viral shedding and hospital stay were defined according to the real-time RT-PCR result at the first admission.
Statistical analysis
The study subjects were grouped by whether supplemental oxygen was required. We present continuous variables as median and interquartile range (IQR) and categorical variables as counts (%). The medians of continuous variables were compared using the Mann–Whitney U test. Proportions for categorical variables were compared using Fisher's exact test. We used Stata (version 13; StataCorp, College Station, TX, USA) for these analyses. For unadjusted comparisons, a two-sided α-value of < 0.05 was considered statistically significant. These analyses were not adjusted for multiple comparisons; given the potential for type I error, the findings should be interpreted as exploratory and descriptive.
Ethics statement
The UUH Institutional Review Board (IRB) approved this study as minimal-risk research because it used data collected for routine clinical practice and waived the requirement for informed consent (IRB File No. 2020-03-030).
DISCUSSION
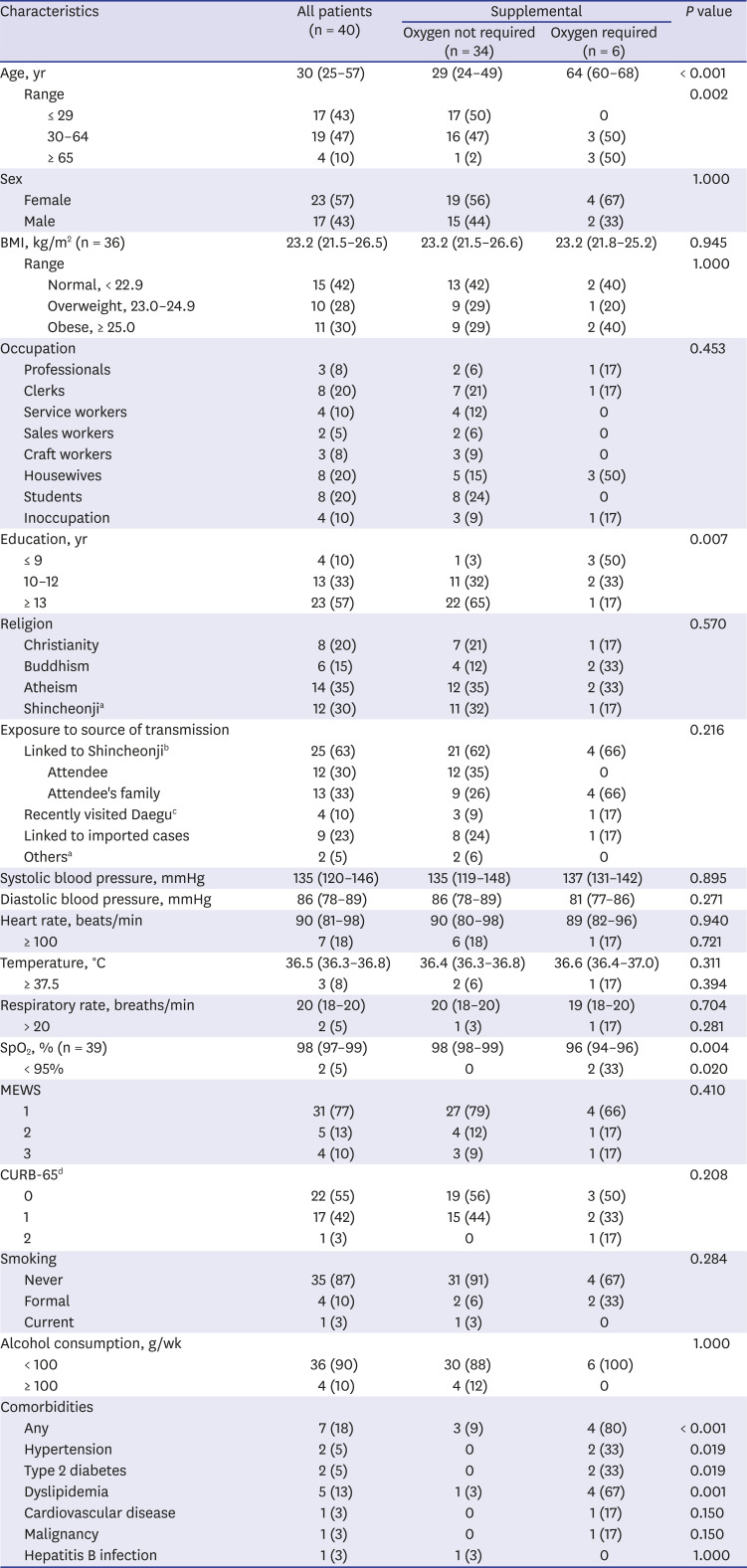
In this retrospective case series of 40 COVID-19 patients admitted to a hospital, only 2 (5%) were asymptomatic and 5 (13%) were presymptomatic. Although this study investigated COVID-19 patients at one hospital, all cases that developed in a metropolitan city of Korea were included; these cases had characteristics similar to those of other COVID-19 patients in Korea.
1617 COVID-19 patients in this study were slightly younger than those in Korea in general (43% vs. 34% aged ≤ 29 years and 18% vs. 24% aged ≥ 60 years).
16 However, females accounted for 58% of the patients (57.6% for all patients with COVID-19 in Korea). The mortality rate was 3% (1/40), which is similar to the 2.3% reported for all patients with COVID-19 in Korea. As of June 20, 2020, the incidence of SARS-CoV-2 infection in Ulsan was 4.6/100,000 people, which is the same as the median value reported in 17 provinces and major cities of Korea (range, 1.1–238.2/100,000 people).
17
The patients requiring supplemental oxygen were older and more likely to have comorbidities than those not requiring supplemental oxygen. The patient who died required supplemental oxygen, was 67 years old, and had cardiovascular disease. This suggests that age and comorbidities are risk factors for poor prognosis.
56
Among the 40 patients in this study, 2 (5%) had asymptomatic SARS-CoV-2 infection. Thirty-three (83%) were symptomatic and five (13%) were presymptomatic. Park et al.
18 reported the epidemiology of the COVID-19 outbreak at a call center in Korea. Among 97 patients, 4 (4%) remained asymptomatic after 14 days of quarantine, which is similar to the 5% reported in our study. Arons et al.
19 reported that the prevalence of asymptomatic infection was 6% (3/48) in residents at a skilled nursing facility in Washington, US, which was similar to the prevalence in the present study. They used 14 symptoms to define symptomatic infections, which were also similar to the 16 symptoms included in the present study. By contrast, many other studies reported much higher prevalence of asymptomatic infection than our study, and they tended to use fewer symptoms to define symptomatic infections.
20212223 An Italian study reported that 42% of infections were asymptomatic when only fever and cough were considered symptoms.
21 In an Icelandic population screening,
20 43% of patients were asymptomatic. However, the authors noted that symptoms almost certainly developed later in some of the asymptomatic patients. Hence, the actual prevalence of asymptomatic infections is likely to be < 43%. Regarding female admitted for delivery in New York city, the asymptomatic infection rate was 88%, which was much higher than that observed in the present study.
22 In that study, the authors considered symptomatic cases to have fever and other symptoms of COVID-19 but did not provide information about the other symptoms. In a study of Greek citizens evacuated from three European countries, asymptomatic infection rate was 88%.
23 The subjects were asked in-flight to complete a paper form with clinical information and received written instructions to report any new symptoms by telephone. The authors did not include symptoms other than fever and cough. Thus, symptoms were likely underestimated in that study. In a review, Oran and Topol
24 reported that asymptomatic SARS-CoV-2 infections accounted for 40%–45% of all infections. However, there were no records of the articles used in their review to confirm whether atypical symptoms such as anorexia, diarrhea, and nausea were evaluated.
24 A COVID-19 infection survey in England published in July 2020 reported that 67% of patients were asymptomatic. However, symptoms were self-reported rather than clinically diagnosed and instances wherein the questions related to symptoms were not answered were defined as asymptomatic.
25 Given these findings, the real prevalence of asymptomatic SARS-CoV-2 infection may be much lower than those reported in previous studies and is associated with the definition of a symptomatic infection and the number of symptoms used to define symptomatic. Therefore, careful history taking with the appropriate number of symptoms is required to diagnose COVID-19.
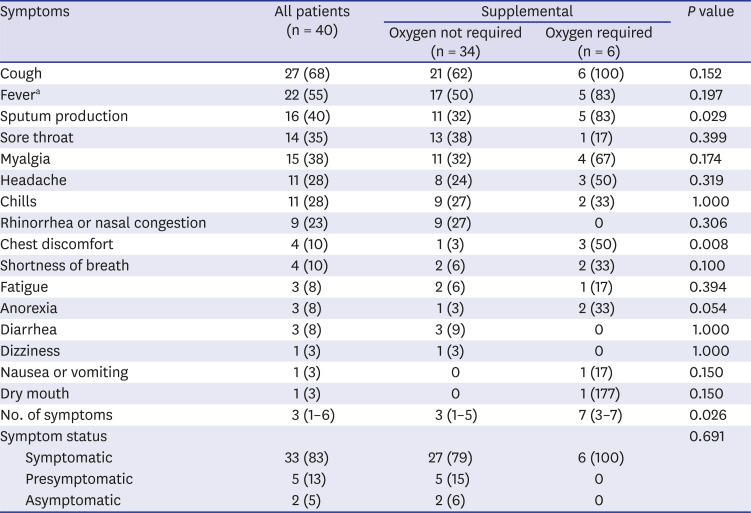
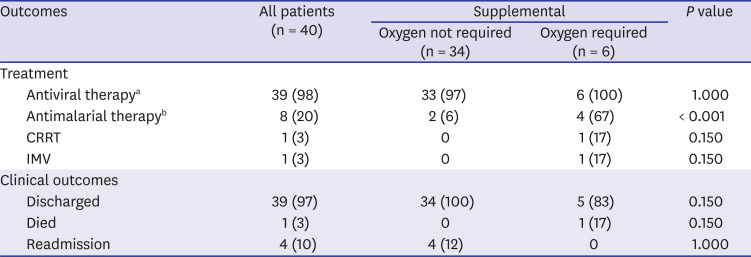
We investigated 16 symptoms of SARS-CoV-2 infection and found that cough and fever were the most common symptoms and that many other symptoms developed in addition to fever or cough. For example, sputum production always developed with cough and shortness of breath always developed with fever. Conversely, myalgia, rhinorrhea or nasal congestion, or diarrhea developed without cough or fever in some patients. Hence, cough, fever, myalgia, rhinorrhea or nasal congestion, and diarrhea were the screening criteria used to detect all cases of symptomatic and presymptomatic SARS-CoV-2 infections. Further studies with varied study populations are required to determine screening criteria with representative symptoms of COVID-19. Sputum production, chest discomfort, and the number of symptoms were associated with requiring supplemental oxygen in univariate analyses. Multivariate analyses are required to confirm whether sputum production, chest discomfort, and a large number of symptoms can predict poor prognosis of COVID-19.
This study has several advantages. First, data were collected from manual medical record reviews and an electronic health record database, thus providing more detailed patient information. Second, we used varied data sources including epidemiological and clinical data identified by a preventive medicine specialist who interviewed all patients, resulting in highly qualified and sufficient information. Finally, we monitored all patients including readmitted patients until they were discharged. Therefore, it was possible to perform continued observation of the natural course of COVID-19.
However, this study has several limitations. First, given the limited number of cases, it was difficult to compare variables using multivariable-adjusted methods. Second, we could only estimate the incubation period for 63% (25/40) of the patients and could not estimate it for the imported cases. The uncertainty of the exact dates might have affected our assessment. Third, we did not have information about the loss of taste or smell as symptoms of COVID-9.
26 There was limited information on the loss of taste or smell as symptoms of COVID-9 in February and March, 2020 when most patients with COVID-19 in this study were hospitalized. Hence, patients’ history of taste or smell was not taken at admission. If loss of taste or smell was included in our screening criteria, the prevalence of asymptomatic SARS-CoV-2 infection might have been < 5% in the present study. Finally, we only investigated the presence of symptoms and did not consider the severity of symptoms. Hence, if a symptom is mild, the clinical importance of the symptom is not clear.
In this case series, we found that the prevalence of asymptomatic SARS-CoV-2 infection was 5%, which is much lower than those previously reported. This finding suggests that the careful investigation of symptom history and follow-up should be performed to detect SARS-CoV-2 infections. Cough, fever, myalgia, rhinorrhea or nasal congestion, and diarrhea were the screening criteria used to cover all symptoms of SARS-CoV-2 infection. Further evaluation with varied study populations is required to create representative screening criteria for COVID-19.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download