INTRODUCTION
METHODS
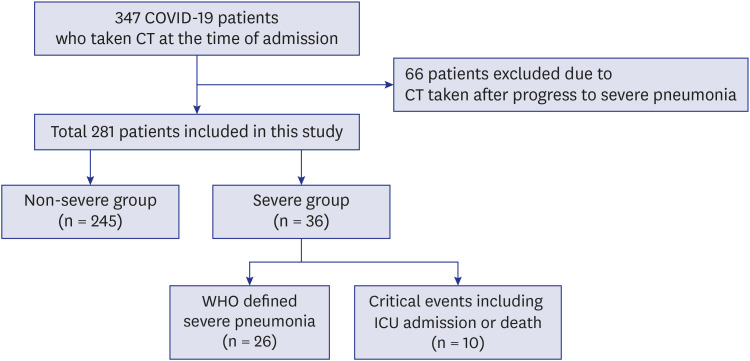
Patients
Chest CT protocol
Image analysis
Statistical analysis
Ethics statement
RESULTS
Patients
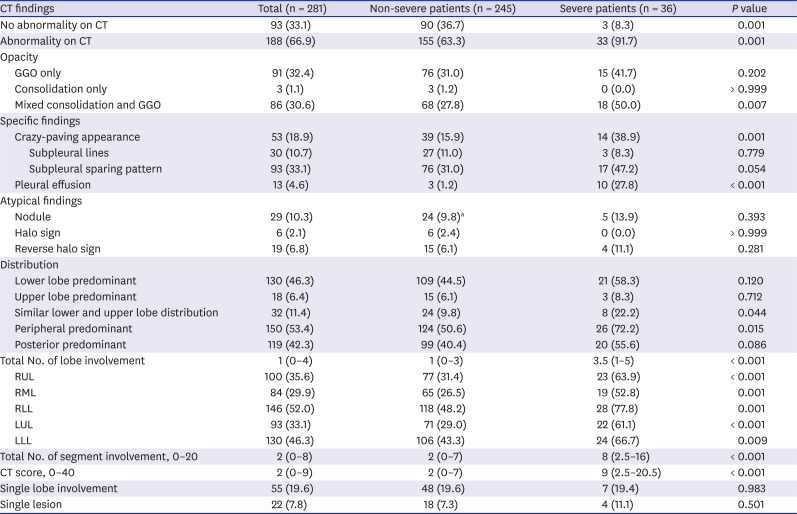
Table 1
Clinical characteristics of coronavirus disease patients
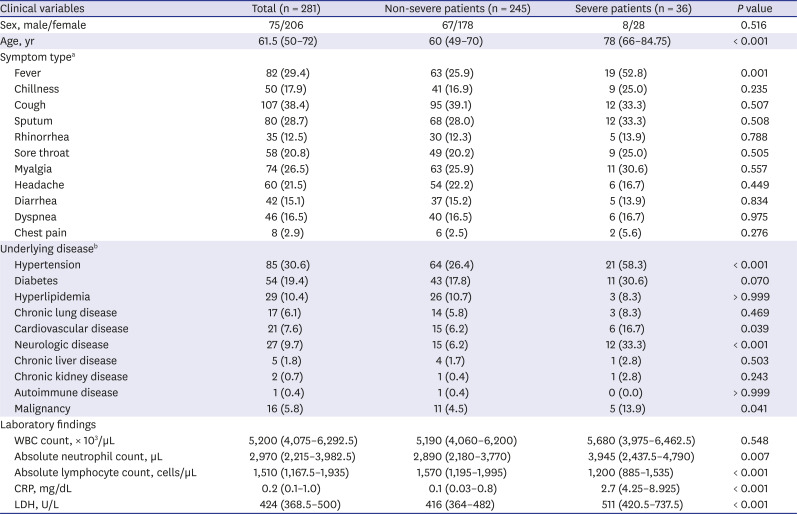
CT findings of severe and non-severe COVID-19 patients
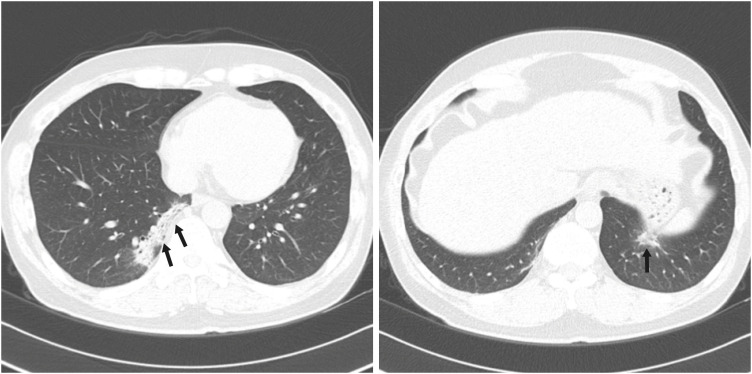 | Fig. 2A 54-year-old male patient with non-severe COVID-19 pneumonia. He had a history of hypertension and ischemic heart disease. Initial chest computed tomography taken one day after admission (arrow) shows a focal peripheral consolidation at both lower lobes. Three segments were involved, and the CT score was 5. There is no pleural effusion. During hospitalization, peripheral oxygen saturation was maintained above 95%.COVID-19 = coronavirus disease 2019, CT = computed tomography.
|
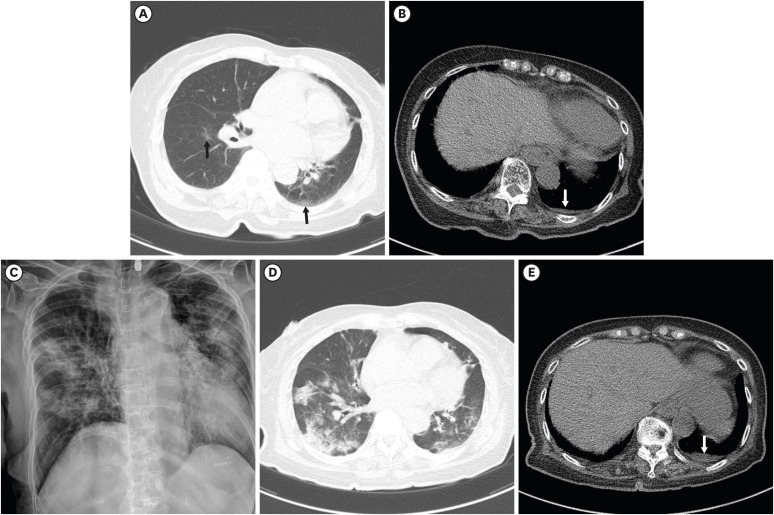 | Fig. 3An 81-year-old female with COVID-19 in the intensive care unit. She had a history of stroke. Initial chest CT one day after admission (A) shows focal GGO at the superior segment of the left lower lobe and the right lower lobe (black arrows), and a small amount of left pleural effusion (B; white arrow). Two segments were involved, and the CT score was 2. Five days after the initial CT, chest radiograph (C) shows an aggravation of the pneumonia. The peripheral oxygen saturation decreased to 88%, and oxygen supply treatment was started. The patient was admitted to the intensive care unit for close monitoring. Follow-up CT (D, E) after treatment showed multifocal consolidation and GGO in both the lungs, and a small amount of left pleural effusion (white arrow) is observed.COVID-19 = coronavirus disease 2019, CT = computed tomography, GGO = ground glass opacity.
|
 | Fig. 5Death of a 92-year-old male with COVID-19. He had a history of stroke, diabetes, and situs inversus totalis. Initial chest CT on the day of admission shows bilateral COVID-19 pneumonia (A, B), and a moderate amount of pleural effusion (C) is observed. All 20 segments were involved, and the CT score was 33. On the next day, peripheral oxygen saturation decreased by 90%. The patient refused active treatment or resuscitation, and died after 15 days due to respiratory failure.COVID-19 = coronavirus disease 2019, CT = computed tomography.
|
Table 2
Comparison of chest CT findings of coronavirus disease patients between two groups
Prognostic imaging and clinical factor for severe COVID-19
Table 3
Univariate and multivariate logistic regression analyses were performed to investigate clinical and CT imaging factors related to severe disease in coronavirus disease patients
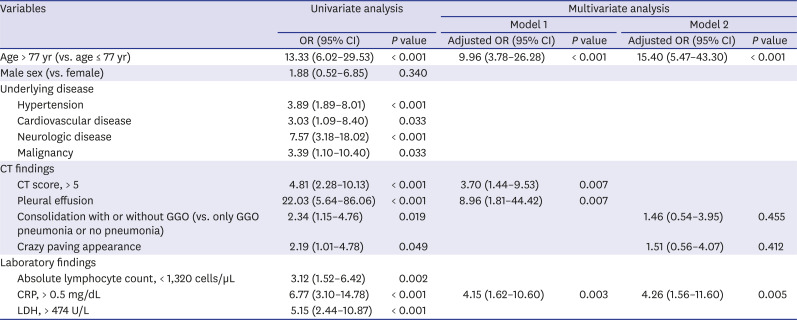
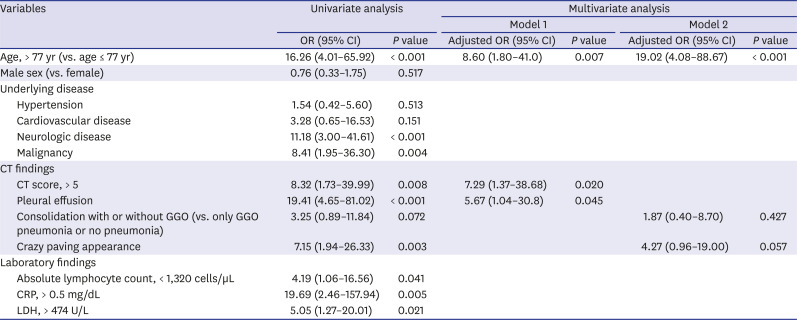
Table 4
Univariate and multivariate logistic regression analyses were performed to investigate clinical and CT imaging factors related to critical events (ICU admission and death) in coronavirus disease patients
DISCUSSION
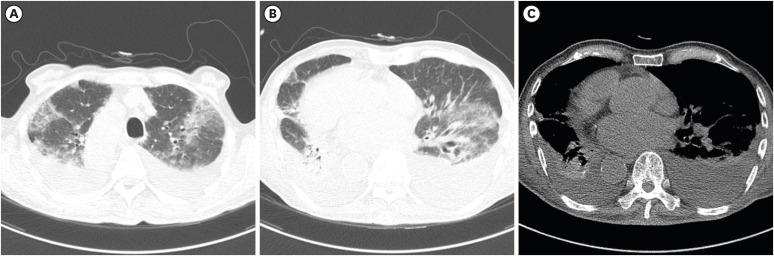
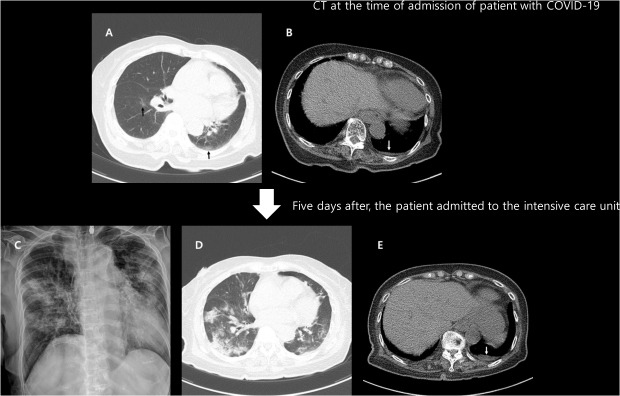
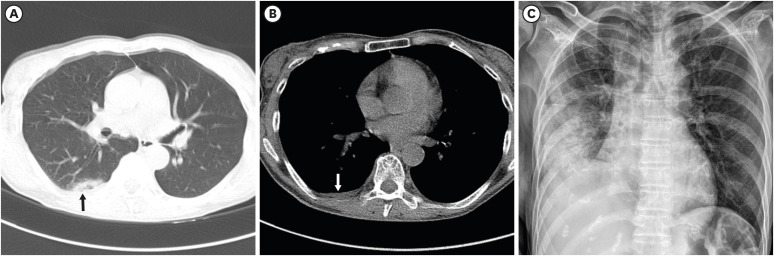 | Fig. 4A 52-year-old female with severe COVID-19 pneumonia. She had a history of hypertension and dementia. An initial chest CT scan on the day of admission shows focal subpleural consolidation (black arrow) with ground-glass opacity at the right lower lobe superior segment (A). A small amount of right pleural effusion is also noted (B; white arrow). One segment was involved, and the CT score was 1. Pneumonia is seen aggravated on the follow-up chest radiograph nine days after the initial CT (C). Peripheral oxygen saturation decreased to 92%, and 2 L nasal prong oxygen therapy was initiated.COVID-19 = coronavirus disease 2019, CT = computed tomography.
|




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download