In Korea, the first case of coronavirus disease 2019 (COVID-19) was confirmed on January 20, 2020. The distinguishing characteristic of COVID-19, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is the substantial transmission potential of asymptomatic or subclinical patients. In a previous study, the viral load of SARS-CoV-2 in the upper respiratory tract of asymptomatic patients was similar to that of symptomatic patients.
1 However, the proportion of asymptomatic patients among the total number of patients with COVID-19 varied between studies. Among patients with mild COVID-19 in community treatment centers, asymptomatic cases accounted for 26.6% (53/199) to 58.7% (371/632).
23
A seroepidemiological investigation can reveal the proportion of asymptomatic or subclinical infections in the general population. In addition, since SARS-CoV-2 is a novel coronavirus, surveillance of antibody seropositivity can provide useful information on the extent of infection and the cumulative herd immunity in the general population.
4 This cross-sectional study on the seroprevalence of antibodies to SARS-CoV-2 included outpatients of university hospitals in Seoul, Korea.
A total of 1,500 serum samples were collected at two hospitals in southwestern Seoul—Korea University Guro Hospital and Hallym University Kangnam Sacred Heart Hospital. In southwestern Seoul, several clusters of COVID-19 outbreaks occurred at churches, sports clubs, and a call center (
Fig. 1).
5 Moreover, a COVID-19 outbreak occurred at a large distribution center in Bucheon City, Gyeonggi-do Province, which is close to southwestern Seoul.
5
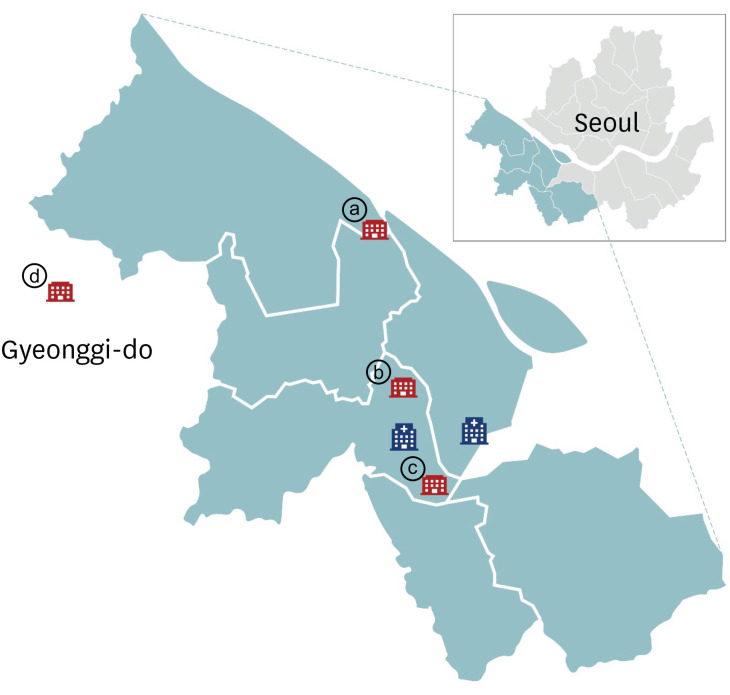 | Fig. 1Map of study site with study hospitals marked in blue. Sites of clusters of coronavirus disease outbreaks near the study hospitals are shown in red: ⓐ sports club, ⓑ call center, ⓒ church, ⓓ distribution center. The COVID-19 outbreak in a sport club (ⓐ) was detected on Jun 5, 2020, and visitors at the sport club from May 28 to June 2, 2020 were investigated. A call center (ⓑ) outbreak was first detected on March 9, 2020. Outbreak in a church (ⓒ) was detected on March 26, 2020 and outbreak at the distribution center (ⓓ) was detected on May 25, 2020.
|
Convenience samples of residual serum samples of outpatients who had visited the hospitals between May 25 and May 29, 2020 were used (
Fig. 2). These patients lived in six administrative districts in southwestern Seoul: Guro-gu, Yeongdeungpo-gu, Gangseo-gu, Yangcheon-gu, Gwanak-gu, and Geumcheon-gu. The serum samples were used anonymously except for the information about sex and age. Serum samples from patients of different ages were collected to investigate the age-stratified seroprevalence of SARS-CoV-2. Samples from patients with laboratory-confirmed COVID-19 were excluded from this study.
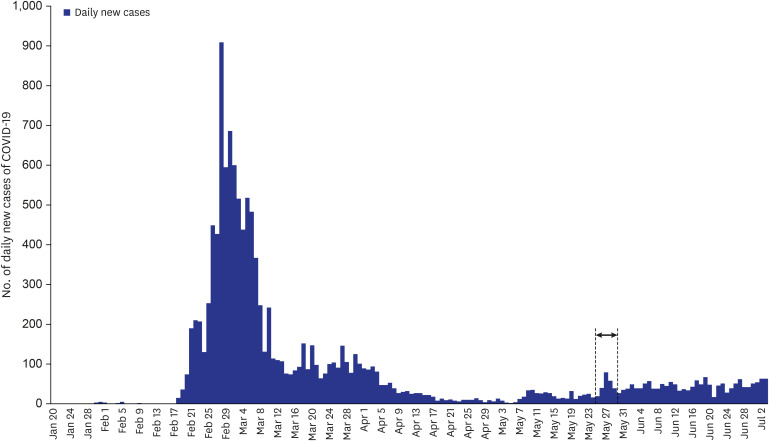 | Fig. 2Number of cases of coronavirus disease as of July 3, 2020 in Korea. Study samples were collected from May 25 to May 29, 2020 (indicated with an arrow).
|
The electrochemiluminescence immunoassay (ECLIA) was performed to detect antibodies including immunoglobulin G (IgG) against SARS-CoV-2 using Elecsys
® Anti-SARS-CoV-2 (Roche, Solna, Switzerland) according to the manufacturer's protocol. Elecsys
® Anti-SARS-CoV-2 used a recombinant protein representing the nucleocapsid antigen of SARS-CoV-2 with a double-antigen sandwich assay.
6 Clinical sensitivity of this assay was reported as 99.5% in polymerase chain reaction (PCR)-confirmed SARS-CoV-2 cases when tested at ≥ 14 days post-PCR confirmation and specificity of Elecsys
® Anti-SARS-CoV-2 was reported as 99.8%.
6 A cutoff index (COI, signal sample/cutoff) of ≥ 1.0 was considered positive for anti-SARS-CoV-2 antibodies. Additionally, a plaque reduction neutralization test (PRNT) was performed for samples that tested positive by ECLIA. Wild-type SARS-CoV-2 (BetaCoV/Korea/KCDC03/2020) was used for PRNT, and the median neutralizing titer (ND50) was measured.
The study population comprised 48.7% men (730/1,500), and the patient age range was 0–92 years. The patients were divided into the following age groups: 0–19 years, 226 (15.1%); 20–29 years, 258 (17.2%); 30–39 years, 261 (17.4%); 40–49 years, 254 (16.9%); 50–59 years, 255 (17.0%); ≥ 60 years, 246 (16.4%). The rate of antibody positivity to SARS-CoV-2 was 0.07% (1 of 1,500; 95% confidence interval [CI], 0–0.44). The COI of a positive sample was 63.82 by ECLIA. ND50 was determined to be 1:764.48 using PRNT. Since the person who showed seropositivity was a 32-year-old man, seroprevalence of antibodies to SARS-CoV-2 was 0.38% (1 of 261; 95% CI, 0.02–2.45) in the 30–39-year age group.
The study hospitals were located at Guro-gu and Yeongdeungpo-gu, Seoul. Among the 25 administrative districts of Seoul, 34% of Chinese immigrants live in these two districts.
7 Since COVID-19 pandemic was first originated from Wuhan, China, concerns about outbreak of SARS-CoV-2 infections had been raised in these areas. However, this study indicated low-level seroprevalence of antibodies to SARS-CoV-2 in southwestern Seoul, Korea. As of May 29, 2020, the incidence of COVID-19 cases among the residents in study sites were as follows: Guro-gu, 0.009% (41/438,308); Yeongdeungpo-gu, 0.008% (34/404,766); Gangseo-gu, 0.007% (44/595,703); Yangcheon-gu, 0.006% (27/460,532); Gwanak-gu, 0.011% (59/516,662); Geumcheon-gu, 0.006% (15/251,370).
8
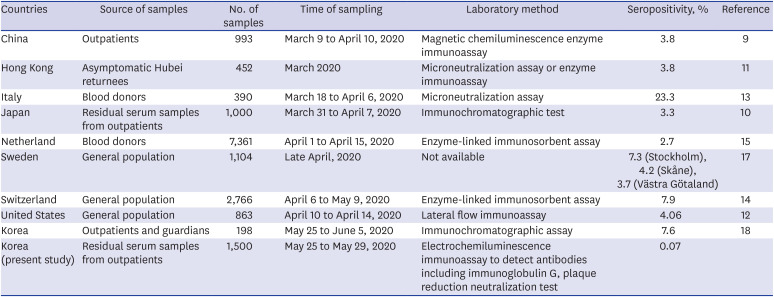
Seroprevalence of antibodies to SARS-CoV-2 varies with country, study population, time of sampling, and laboratory testing method (
Table 1). In cross-sectional studies conducted in outpatients in China and Japan, the seropositivity rate of antibodies to SARS-CoV-2 was 3.8% and 3.3%, respectively.
910 In another study, seropositivity against SARS-CoV-2 was evaluated in Hong Kong residents who were evacuated from Hubei Province, China, on March 4–5, 2020; the analysis of serum samples obtained within 13 days after evacuation revealed that 17 of 452 (3.8%) individuals were seropositive.
11 In a community survey in California, the United States, the seroprevalence of antibodies to SARS-CoV-2 was 4.06%.
12 In Lombardy, Italy, where the first COVID-19 case was identified on February 20, 2020, the seropositivity rate of neutralizing antibody against SARS-CoV-2 in blood donors was 23.3% (91/390). A seroepidemiological investigation revealed that 1.7% (5/300) of the blood donors collected before identification of the first case tested seropositive against SARS-CoV-2.
13 In a population-based serosurveillance study in Switzerland, 7.9% of the population showed anti-SARS-CoV-2 IgG antibodies.
14 In particular, the seropositivity rate was highest in the 20–49 year age group (9.9%). In the Netherlands, the seroprevalence of antibodies to SARS-CoV-2 was 2.7% among 7,361 blood donors, and seropositivity in donors aged 18–30 years was significantly higher (4.2%) than that in other age groups.
15 The Swedish response to the COVID-19 pandemic aimed to promote herd immunity by allowing a proportion of the population to be infected rather than containing the virus.
16 Nevertheless, the seroprevalence of SARS-CoV-2 antibodies was just 7.3% in Stockholm, Sweden.
17 The seropositive rate of SARS-CoV-2 was higher in young adults than in the elderly in Sweden—4.7%, 6.7%, and 2.7% in the age group of 0–19 years, 20–64 years; and 65–95 years, respectively.
17
Table 1
Studies on the seroprevalence of coronavirus disease 2019
|
Countries |
Source of samples |
No. of samples |
Time of sampling |
Laboratory method |
Seropositivity, % |
Reference |
|
China |
Outpatients |
993 |
March 9 to April 10, 2020 |
Magnetic chemiluminescence enzyme immunoassay |
3.8 |
9
|
|
Hong Kong |
Asymptomatic Hubei returnees |
452 |
March 2020 |
Microneutralization assay or enzyme immunoassay |
3.8 |
11
|
|
Italy |
Blood donors |
390 |
March 18 to April 6, 2020 |
Microneutralization assay |
23.3 |
13
|
|
Japan |
Residual serum samples from outpatients |
1,000 |
March 31 to April 7, 2020 |
Immunochromatographic test |
3.3 |
10
|
|
Netherland |
Blood donors |
7,361 |
April 1 to April 15, 2020 |
Enzyme-linked immunosorbent assay |
2.7 |
15
|
|
Sweden |
General population |
1,104 |
Late April, 2020 |
Not available |
7.3 (Stockholm), 4.2 (Skåne), 3.7 (Västra Götaland) |
17
|
|
Switzerland |
General population |
2,766 |
April 6 to May 9, 2020 |
Enzyme-linked immunosorbent assay |
7.9 |
14
|
|
United States |
General population |
863 |
April 10 to April 14, 2020 |
Lateral flow immunoassay |
4.06 |
12
|
|
Korea |
Outpatients and guardians |
198 |
May 25 to June 5, 2020 |
Immunochromatographic assay |
7.6 |
18
|
|
Korea (present study) |
Residual serum samples from outpatients |
1,500 |
May 25 to May 29, 2020 |
Electrochemiluminescence immunoassay to detect antibodies including immunoglobulin G, plaque reduction neutralization test |
0.07 |
|

In Korea, seroprevalence of antibodies to SARS-CoV-2 among 198 outpatients and their guardians was 7.6% in Daegu in which more than half (56.9%, 6,881/12,088) of domestic cases with COVID-19 occurred as of August 11, 2020.
18 However, because of the heterogeneous and inconsistent results, the clinical performance of SARS-CoV-2 rapid lateral flow assay is still under evaluation, requiring confirmatory testing such as neutralization assay.
19 Considering the possible cross-reactivity from the pre-existing common cold coronaviruses (NL63, 229E, OC43 or HKU1) antibodies, it is necessary to be careful in interpreting the results.
This study has some limitations. First, a selection bias could have existed; hence, the study result might not represent the seroprevalence of antibodies to SARS-CoV-2 in the general population of southwestern Seoul, Korea. Second, epidemiological information of one person who was seropositive was not available. Third, this study was a one-time cross-sectional investigation, and seroprevalence may change as the pandemic progresses. Thus, a cross-sectional investigation should be repeated to estimate the herd immunity level.
However, this study revealed low seroprevalence of antibodies to SARS-CoV-2 in healthcare facility users in Seoul, Korea. A low seroprevalence (0.07%) reflects that the COVID-19 pandemic is relatively under control by implementation of social distancing and rigorous epidemiological investigations, including contact tracing, active testing, and patient isolation. However, low seroprevalence of SARS-CoV-2 suggests that most people are still vulnerable to SARS-CoV-2 infection. Considering the basic reproduction number of COVID-19, > 60% of the population should be immune against SARS-CoV-2 to suppress the pandemic. Therefore, we are still at the beginning of the pandemic, and we should follow social distancing norms to prevent the explosive spread of COVID-19 until effective vaccines are available. Serosurveillance should be repeated to estimate the level of herd immunity in each country as the pandemic progress.
Ethics Statement
The study protocol was exempted from review by the Public Institutional Review Board designated by the Ministry of Health and Welfare (approval No. P01-202005-31-006).
Go to :







 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download