1. Mohr JF, Murray BE. Point: vancomycin is not obsolete for the treatment of infection caused by methicillin-resistant
Staphylococcus aureus
. Clin Infect Dis. 2007; 44(12):1536–1542. PMID:
17516395.
2. Minejima E, Choi J, Beringer P, Lou M, Tse E, Wong-Beringer A. Applying new diagnostic criteria for acute kidney injury to facilitate early identification of nephrotoxicity in vancomycin-treated patients. Antimicrob Agents Chemother. 2011; 55(7):3278–3283. PMID:
21576448.

3. Cadle RM, Mansouri MD, Darouiche RO. Vancomycin-induced elevation of liver enzyme levels. Ann Pharmacother. 2006; 40(6):1186–1189. PMID:
16720708.

4. Forouzesh A, Moise PA, Sakoulas G. Vancomycin ototoxicity: a reevaluation in an era of increasing doses. Antimicrob Agents Chemother. 2009; 53(2):483–486. PMID:
19001107.

5. Rello J, Sole-Violan J, Sa-Borges M, Garnacho-Montero J, Muñoz E, Sirgo G, et al. Pneumonia caused by oxacillin-resistant
Staphylococcus aureus treated with glycopeptides. Crit Care Med. 2005; 33(9):1983–1987. PMID:
16148469.
6. Rybak M, Lomaestro B, Rotschafer JC, Moellering R Jr, Craig W, Billeter M, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009; 66(1):82–98. PMID:
19106348.
7. Bakke V, Sporsem H, Von der Lippe E, Nordøy I, Lao Y, Nyrerød HC, et al. Vancomycin levels are frequently subtherapeutic in critically ill patients: a prospective observational study. Acta Anaesthesiol Scand. 2017; 61(6):627–635. PMID:
28444760.

8. Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003; 139(2):137–147. PMID:
12859163.

9. Matzke GR, McGory RW, Halstenson CE, Keane WF. Pharmacokinetics of vancomycin in patients with various degrees of renal function. Antimicrob Agents Chemother. 1984; 25(4):433–437. PMID:
6732213.

10. Matzke GR, Zhanel GG, Guay DR. Clinical pharmacokinetics of vancomycin. Clin Pharmacokinet. 1986; 11(4):257–282. PMID:
3530582.

11. Golper TA, Noonan HM, Elzinga L, Gilbert D, Brummett R, Anderson JL, et al. Vancomycin pharmacokinetics, renal handling, and nonrenal clearances in normal human subjects. Clin Pharmacol Ther. 1988; 43(5):565–570. PMID:
3365918.

12. Lubran MM. Renal function in the elderly. Ann Clin Lab Sci. 1995; 25(2):122–133. PMID:
7785962.
13. MacDiarmid SA, McIntyre WJ, Anthony A, Bailey RR, Turner JG, Arnold EP. Monitoring of renal function in patients with spinal cord injury. BJU Int. 2000; 85(9):1014–1018. PMID:
10848686.

14. Verhave JC, Fesler P, Ribstein J, du Cailar G, Mimran A. Estimation of renal function in subjects with normal serum creatinine levels: influence of age and body mass index. Am J Kidney Dis. 2005; 46(2):233–241. PMID:
16112041.

15. Delanaye P, Mariat C, Cavalier E, Maillard N, Krzesinski JM, White CA. Trimethoprim, creatinine and creatinine-based equations. Nephron Clin Pract. 2011; 119(3):c187–c193. PMID:
21832843.

16. Andreev E, Koopman M, Arisz L. A rise in plasma creatinine that is not a sign of renal failure: which drugs can be responsible? J Intern Med. 1999; 246(3):247–252. PMID:
10475992.

17. Maggi P, Montinaro V, Mussini C, Di Biagio A, Bellagamba R, Bonfanti P, et al. Novel antiretroviral drugs and renal function monitoring of HIV patients. AIDS Rev. 2014; 16(3):144–151. PMID:
25102336.
18. Tanaka A, Aiba T, Otsuka T, Suemaru K, Nishimiya T, Inoue T, et al. Population pharmacokinetic analysis of vancomycin using serum cystatin C as a marker of renal function. Antimicrob Agents Chemother. 2010; 54(2):778–782. PMID:
19933799.

19. Filler G, Bökenkamp A, Hofmann W, Le Bricon T, Martínez-Brú C, Grubb A. Cystatin C as a marker of GFR--history, indications, and future research. Clin Biochem. 2005; 38(1):1–8. PMID:
15607309.

20. Abrahamson M, Olafsson I, Palsdottir A, Ulvsbäck M, Lundwall A, Jensson O, et al. Structure and expression of the human cystatin C gene. Biochem J. 1990; 268(2):287–294. PMID:
2363674.

21. Jacobsson B, Lignelid H, Bergerheim US. Transthyretin and cystatin C are catabolized in proximal tubular epithelial cells and the proteins are not useful as markers for renal cell carcinomas. Histopathology. 1995; 26(6):559–564. PMID:
7665147.

22. Murty MSN, Sharma UK, Pandey VB, Kankare SB. Serum cystatin C as a marker of renal function in detection of early acute kidney injury. Indian J Nephrol. 2013; 23(3):180–183. PMID:
23814415.

23. Shlipak MG, Mattes MD, Peralta CA. Update on cystatin C: incorporation into clinical practice. Am J Kidney Dis. 2013; 62(3):595–603. PMID:
23701892.

24. Roos JF, Doust J, Tett SE, Kirkpatrick CMJ. Diagnostic accuracy of cystatin C compared to serum creatinine for the estimation of renal dysfunction in adults and children--a meta-analysis. Clin Biochem. 2007; 40(5-6):383–391. PMID:
17316593.

25. Rebollo N, Cepeda-Piorno FJ. Cystatin C for therapeutic drug monitoring. Clin Chem. 2015; 61(6):804–807. PMID:
26025947.

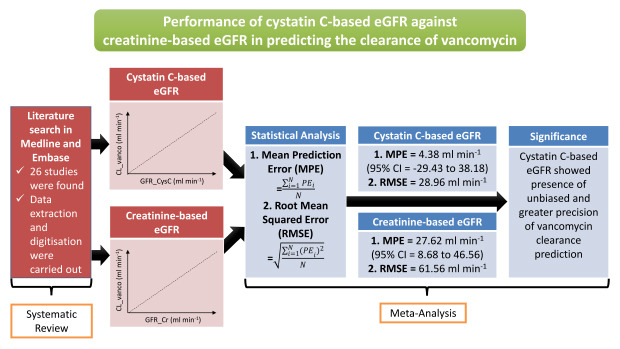
26. Sheiner LB, Beal SL. Some suggestions for measuring predictive performance. J Pharmacokinet Biopharm. 1981; 9(4):503–512. PMID:
7310648.

27. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003; 327(7414):557–560. PMID:
12958120.

28. Borenstein M, Hedges L, Higgins J, Rothstein H. Introduction to Meta-Analysis. Chichester: John Wiley & Sons, Ltd.;2009.
29. Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000; 56(2):455–463. PMID:
10877304.

30. Birt JK, Chandler MH. Using clinical data to determine vancomycin dosing parameters. Ther Drug Monit. 1990; 12:206–209. PMID:
2315979.
31. Polard E, Le Bouquin V, Le Corre P, Kérebel C, Trout H, Feuillu A, et al. Non steady state and steady state PKS Bayesian forecasting and vancomycin pharmacokinetics in ICU adult patients. Ther Drug Monit. 1999; 21(4):395–403. PMID:
10442692.

32. Yoshida M, Yasuda N, Nishikata M, Okamoto K, Uchida T, Matsuyama K. New recommendations for vancomycin dosage for patients with MRSA pneumonia with various degrees of renal function impairment. J Infect Chemother. 2005; 11(4):182–188. PMID:
16133709.

33. Okamoto G, Sakamoto T, Kimura M, Ukishima Y, Sonoda A, Mori N, et al. Serum cystatin C as a better marker of vancomycin clearance than serum creatinine in elderly patients. Clin Biochem. 2007; 40(7):485–490. PMID:
17336280.

34. Omote S, Yano Y, Hashida T, Masuda S, Yano I, Katsura T, et al. A retrospective analysis of vancomycin pharmacokinetics in Japanese cancer and non-cancer patients based on routine trough monitoring data. Biol Pharm Bull. 2009; 32(1):99–104. PMID:
19122288.

35. Pea F, Furlanut M, Negri C, Pavan F, Crapis M, Cristini F, et al. Prospectively validated dosing nomograms for maximizing the pharmacodynamics of vancomycin administered by continuous infusion in critically ill patients. Antimicrob Agents Chemother. 2009; 53(5):1863–1867. PMID:
19223642.

36. Yamamoto M, Kuzuya T, Baba H, Yamada K, Nabeshima T. Population pharmacokinetic analysis of vancomycin in patients with gram-positive infections and the influence of infectious disease type. J Clin Pharm Ther. 2009; 34(4):473–483. PMID:
19583681.

37. Dolton M, Xu H, Cheong E, Maitz P, Kennedy P, Gottlieb T, et al. Vancomycin pharmacokinetics in patients with severe burn injuries. Burns. 2010; 36(4):469–476. PMID:
19875238.

38. Kees MG, Hilpert JW, Gnewuch C, Kees F, Voegeler S. Clearance of vancomycin during continuous infusion in Intensive Care Unit patients: correlation with measured and estimated creatinine clearance and serum cystatin C. Int J Antimicrob Agents. 2010; 36(6):545–548. PMID:
20863668.

39. Jeurissen A, Sluyts I, Rutsaert R. A higher dose of vancomycin in continuous infusion is needed in critically ill patients. Int J Antimicrob Agents. 2011; 37(1):75–77. PMID:
21074374.

40. Lee JP, Dang AT. Evaluation of methods to estimate glomerular filtration rate versus actual drug clearance in patients with chronic spinal cord injury. Spinal Cord. 2011; 49(12):1158–1163. PMID:
21788951.

41. Shimamoto Y, Fukuda T, Tominari S, Fukumoto K, Ueno K, Dong M, et al. Decreased vancomycin clearance in patients with congestive heart failure. Eur J Clin Pharmacol. 2013; 69(3):449–457. PMID:
22791272.

42. Shimamoto Y, Fukuda T, Tanaka K, Komori K, Sadamitsu D. Systemic inflammatory response syndrome criteria and vancomycin dose requirement in patients with sepsis. Intensive Care Med. 2013; 39(7):1247–1252. PMID:
23604132.

43. Adane ED, Herald M, Koura F. Pharmacokinetics of vancomycin in extremely obese patients with suspected or confirmed
Staphylococcus aureus infections. Pharmacotherapy. 2015; 35(2):127–139. PMID:
25644478.
44. Nielsen HE, Hansen HE, Korsager B, Skov PE. Renal excretion of vancomycinin in kidney disease. Acta Med Scand. 1975; 197(4):261–264. PMID:
1136852.
45. Blouin RA, Bauer LA, Miller DD, Record KE, Griffen WO. Vancomycin pharmacokinetics in normal and morbidly obese subjects. Antimicrob Agents Chemother. 1982; 21(4):575–580. PMID:
7081978.

46. Rotschafer JC, Crossley K, Zaske DE, Mead K, Sawchuk RJ, Solem LD. Pharmacokinetics of vancomycin: observations in 28 patients and dosage recommendations. Antimicrob Agents Chemother. 1982; 22(3):391–394. PMID:
7137982.

47. Garaud JJ, Regnier B, Inglebert F, Faurisson F, Bauchet J, Vachon F. Vancomycin pharmacokinetics in critically ill patients. J Antimicrob Chemother. 1984; 14 Suppl D:53–57.

48. Brater DC, Bawdon RE, Anderson SA, Purdue GF, Hunt JL. Vancomycin elimination in patients with burn injury. Clin Pharmacol Ther. 1986; 39(6):631–634. PMID:
3709027.

49. Taber DJ, Fann AL, Malat G, Dupuis RE. Evaluation of estimated and measured creatinine clearances for predicting the pharmacokinetics of vancomycin in adult liver transplant recipients. Ther Drug Monit. 2003; 25(1):67–72. PMID:
12548147.

50. Dailly E, Le Floch R, Deslandes G, Pannier M, Jolliet P. Influence of glomerular filtration rate on the clearance of vancomycin administered by continuous infusion in burn patients. Int J Antimicrob Agents. 2008; 31(6):537–539. PMID:
18462925.

51. Park SJ, Yang JH, Park HJ, In YW, Lee YM, Cho YH, et al. Trough concentrations of vancomycin in patients undergoing extracorporeal membrane oxygenation. PLoS One. 2015; 10(11):e0141016. PMID:
26544074.

52. Baptista JP, Roberts JA, Sousa E, Freitas R, Deveza N, Pimentel J. Decreasing the time to achieve therapeutic vancomycin concentrations in critically ill patients: developing and testing of a dosing nomogram. Crit Care. 2014; 18(6):654. PMID:
25475123.

53. Shin JE, Lee SM, Eun HS, Park MS, Park KI, Namgung R. Usefulness of serum cystatin C to determine the dose of vancomycin in neonate. Korean J Pediatr. 2015; 58(11):421–426. PMID:
26692877.

54. Chen YC, Feng JF, Li B, Zhang L, Yang YW. Estimation of safe and effective dose of vancomycin in MRSA-infected patients using serum cystatin C concentrations. Int J Clin Pharmacol Ther. 2013; 51(3):161–169. PMID:
23195915.

55. Barreto EF, Rule AD, Murad MH, Kashani KB, Lieske JC, Erwin PJ, et al. Prediction of the renal elimination of drugs with cystatin C vs creatinine: a systematic review. Mayo Clin Proc. 2019; 94(3):500–514. PMID:
30713050.

56. Matzke GR, Aronoff GR, Atkinson AJ Jr, Bennett WM, Decker BS, Eckardt KU, et al. Drug dosing consideration in patients with acute and chronic kidney disease-a clinical update from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2011; 80(11):1122–1137. PMID:
21918498.

57. Huang SH, Macnab JJ, Sontrop JM, Filler G, Gallo K, Lindsay RM, et al. Performance of the creatinine-based and the cystatin C-based glomerular filtration rate (GFR) estimating equations in a heterogenous sample of patients referred for nuclear GFR testing. Transl Res. 2011; 157(6):357–367. PMID:
21575920.

58. Zhang Z, Lu B, Sheng X, Jin N. Cystatin C in prediction of acute kidney injury: a systemic review and meta-analysis. Am J Kidney Dis. 2011; 58(3):356–365. PMID:
21601330.

59. Jin SJ, Bae SC, Kim HW, Kim HK, Na EJ, Ahn BS, et al. Evaluation of the effect of initial dose of vancomycin using serum cystatin C as a marker in elderly patients. Infect Chemother. 2009; 41(4):224–229.

60. Brou NA, Jacqz-Aigrain E, Zhao W. Cystatin C as a potential biomarker for dosing of renally excreted drugs. Br J Clin Pharmacol. 2015; 80(1):20–27. PMID:
25655191.

61. Tanaka A, Suemaru K, Otsuka T, Ido K, Nishimiya T, Sakai I, et al. Estimation of the initial dose setting of vancomycin therapy with use of cystatin C as a new marker of renal function. Ther Drug Monit. 2007; 29(2):261–264. PMID:
17417082.

62. Yim DS. Overestimation of vancomycin clearance by the linear regression formula in Rodvold's report: why? Infect Chemother. 2014; 46(1):62–63. PMID:
24693474.

63. Garrelts JC, Godley PJ, Horton MW, Karboski JA. Accuracy of Bayesian, Sawchuk-Zaske, and nomogram dosing methods for vancomycin. Clin Pharm. 1987; 6(10):795–799. PMID:
3505841.
64. Burton ME, Gentle DL, Vasko MR. Evaluation of a Bayesian method for predicting vancomycin dosing. DICP. 1989; 23(4):294–300. PMID:
2728513.

65. Grubbs FE. Procedures for detecting outlying observations in samples. Technometrics. 1969; 11(1):1–21.

66. Viechtbauer W, Cheung MW. Outlier and influence diagnostics for meta-analysis. Res Synth Methods. 2010; 1(2):112–125. PMID:
26061377.

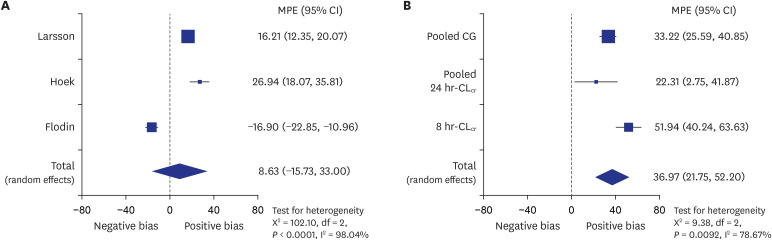
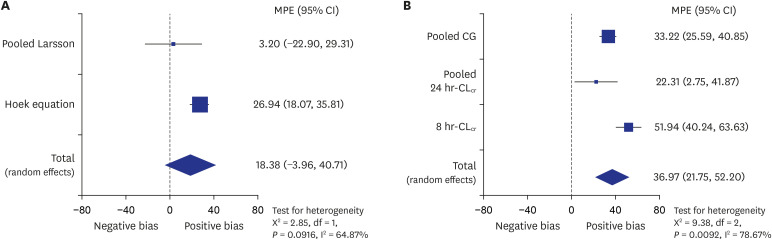
67. Flodin M, Jonsson AS, Hansson LO, Danielsson LA, Larsson A. Evaluation of gentian cystatin C reagent on Abbott Ci8200 and calculation of glomerular filtration rate expressed in mL/min/1.73 m
2 from the cystatin C values in mg/L. Scand J Clin Lab Invest. 2007; 67(5):560–567. PMID:
17763193.
68. Hoek FJ, Kemperman FAW, Krediet RT. A comparison between cystatin C, plasma creatinine and the Cockcroft and Gault formula for the estimation of glomerular filtration rate. Nephrol Dial Transplant. 2003; 18(10):2024–2031. PMID:
13679476.

69. Larsson A, Malm J, Grubb A, Hansson LO. Calculation of glomerular filtration rate expressed in mL/min from plasma cystatin C values in mg/L. Scand J Clin Lab Invest. 2004; 64(1):25–30. PMID:
15025426.

70. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976; 16(1):31–41. PMID:
1244564.

71. Schwartz GJ, Haycock GB, Edelmann CM Jr, Spitzer A. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics. 1976; 58(2):259–263. PMID:
951142.




 PDF
PDF Citation
Citation Print
Print



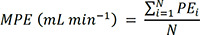
 XML Download
XML Download