This article has been
cited by other articles in ScienceCentral.
Abstract
Nephrogenic systemic fibrosis (NSF) is a progressive systemic fibrosing disease that may occur after gadolinium contrast exposure. It can lead to severe complications and even death. NSF is highly prevalent among patients with advanced chronic kidney disease (CKD). In this report, however, we describe the case of a patient with NSF that occurred during early CKD. A 65-year-old man with stage 3a CKD was transferred to our hospital because of lower extremity edema. The medical history revealed that he was exposed to gadolinium 185 days earlier, and the result of his tibial skin biopsy was consistent with NSF. The patient underwent a combined therapy with ultraviolet-A1 phototherapy and methotrexate and steroid therapy for 6 months. The combined therapy stopped the systemic progression of NSF.
Keywords: Nephrogenic Systemic Fibrosis, Chronic Kidney Disease, Gadolinium-based Contrast Agents
INTRODUCTION
Nephrogenic systemic fibrosis (NSF) is a rare disease that presents with skin sclerosis and joint contracture after exposure to gadolinium-based contrast agents (GBCAs), which are used during magnetic resonance imaging (MRI). NSF can result in death when the fibrosis expands systemically to the heart, lung, and diaphragm.
1 Although the development of NSF varies according to kidney function, the type and dose of GBCAs, NSF mainly occurs in patients with advanced chronic kidney disease (CKD), especially CKD stages 4 and 5.
Three cases of NSF have been reported in Korea. The patients in two of the cases were undergoing dialysis while the patient in the last case had acute kidney injury (AKI) and stage 3b CKD.
234 We present a case involving an NSF that occurred in a patient with stage 3a CKD following gadoterate meglumine (type 2 GBCAs, macrocyclic ligand) administration. The systemic progression of NSF in the patient stopped when he underwent ultraviolet (UV)-A1 phototherapy and methotrexate (MTX) and steroid therapy.
CASE DESCRIPTION
A 65-year-old man was referred to the hospital in 5th July 2019 because of refractory edema in both legs.
The patient had a history of CKD stage 3a, a hypotrophic left kidney, and hypertension. He had been prescribed amlodipine 2.5 mg for hypertensive medication. He also underwent radical prostatectomy for prostate cancer two years earlier. He underwent pelvic MRI using gadoterate meglumine (15 mL), immediately after iodide contrast-enhanced chest and abdomen computed tomography (CT) for cancer surveillance. Thirty days before CT scan, his creatinine level was 1.28 mg/dL and his estimated glomerular filtration rate (eGFR) was 58 mL/min/1.73 m
2.
5 After 185 days, he visited a local clinic because of edematous changes in both lower extremities. CT angiography of the lower extremities was performed; no stenosis or occlusion of vessels was observed. Although diuretics were administered for 2 weeks, the edema worsened and was accompanied by pruritus and pain (
Fig. 1). Eventually, he was referred to our hospital for further evaluation.
Fig. 1
Change in renal function and clinical course in our patient. The horizontal axis illustrates the chronological manifestation in the patient following gadolinium exposure. The vertical axis indicates patient renal function in terms of serum creatinine level. The eGFR is presented in brackets.
eGFR = estimated glomerular filtration rate.
aMeasurement in mg/dL (mL/min/1.73 m2).
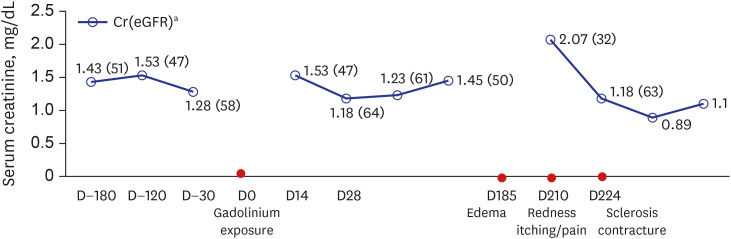
Upon admission, his body temperature was 36.8°C and his blood pressure was 110/60 mmHg. His height was 161.3 cm, weight was 53.2 kg and body mass index was 20.4 kg/m2. Physical examination revealed grade 2+ pretibial pitting edema with redness, mild heat, and tenderness in both lower extremities. Both inguinal lymph nodes were not enlarged.
Initial laboratory findings at admission (about 210 days after MRI scan) were as follows: white blood cell count, 6,200 /μL (neutrophil 66%, lymphocyte 22%, monocyte 10.3%, eosinophil 0.5%); hemoglobin, 10.7 g/dL; platelet, 253,000 /μL; blood urea nitrogen, 41 mg/dL; serum creatinine, 2.07 mg/dL; eGFR, 32 mL/min/1.73 m2; serum sodium, 134 mmol/L; potassium, 3.4 mmol/L; chloride, 102 mmol/L; total CO2, 20 mmol/L; calcium, 9.3 mg/dL; phosphate, 1.5 mg/dL; total protein, 6.9 g/dL; albumin, 4.0 g/dL; aspartate transaminase, 43 U/L; alanine aminotransferase, 15 U/L; and C-reactive protein, 0.96 mg/dL. Routine urine analysis revealed a pH of 6.0 and trace proteins. Red and white blood cells were absent in the urine. The spot urine protein/creatinine ratio was 209 mg/g and the spot urine albumin/creatinine ratio was 60 mg/g. Thyroid function tests were normal: thyroid stimulating hormone, 2.47 μIU/mL and free T4, 0.98 ng/dL. Fractional excretion of Na 0.1% and Fractional excretion of urea on admission was 17.6%, which suggested the patient was prerenal AKI.
Chest radiography and echocardiogram findings were normal. During doppler sonography of both lower extremities, the venous velocity was normal and there was no evidence of thrombosis. None of the aforementioned findings were suggestive of refractory edema.
Fluid therapy was administered to treat the AKI, which was probably caused by the diuretics he was given during his two-week stay in the local clinic. Subsequently, his renal function improved: serum creatinine, 1.18 mg/dL; and eGFR, 63.9 mL/min/1.73 m
2. Since the redness and tenderness of both legs were suggestive of cellulitis-associated edema, intravenous cefazolin was administered for two weeks. However, the lesion gradually spread from his ankle to his thigh and changed into fibrotic induration. Contracture of the ankle and knee joints also occurred (
Fig. 2).
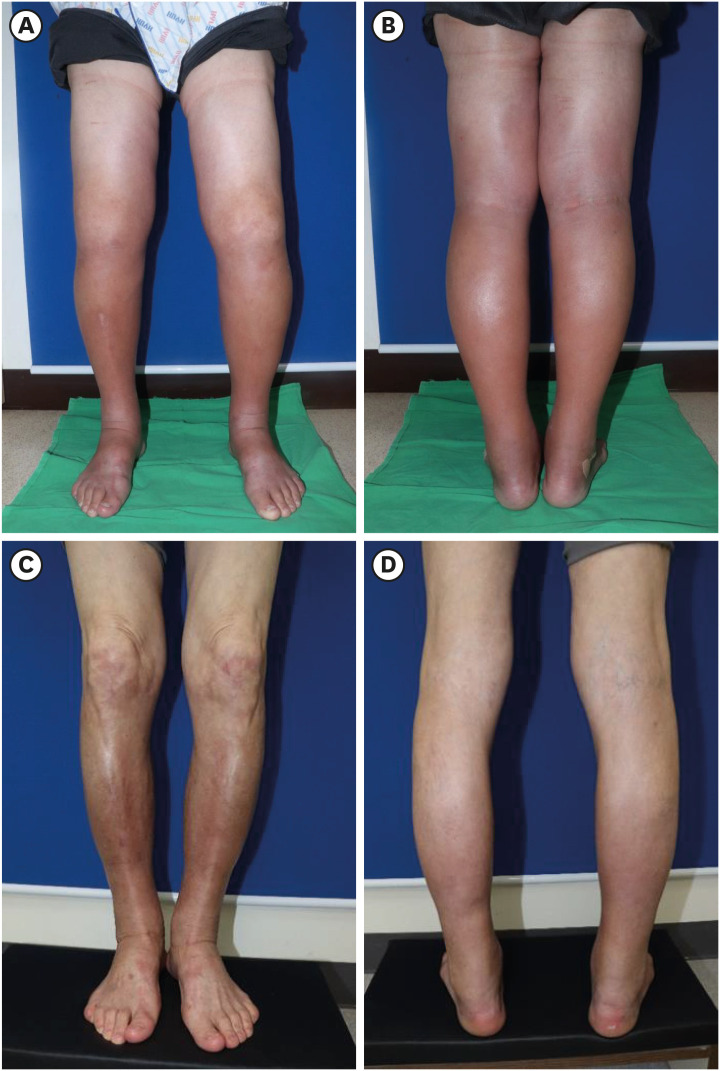
Fig. 2
Clinical images of the lower extremities before treatment and six months after treatment. (A, B) Symmetrical edema with erythematous papules extending from the dorsum to the thigh. (C, D) Contracture of both ankles and hyperpigmentation of both anterior tibial lesions.
The images are published under informed consent of the patient.
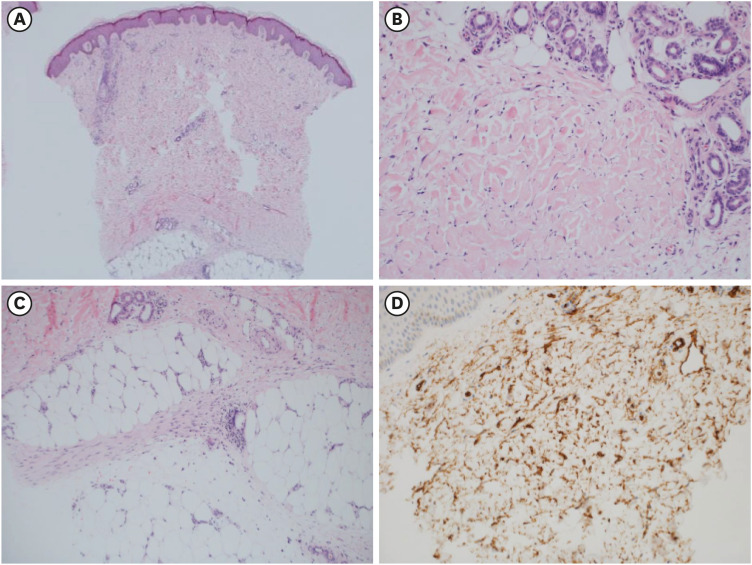
To detect diffuse or localized systemic sclerosis, antinuclear antibody, anti-Scl 70 antibody, and anti-centromere antibody tests were performed, but the results were all negative. Also, eosinophilia and paraproteinemia was not seen, which is commonly accompanied in scleromyxedema or eosinophilic fasciitis. Although approximately 50 weeks had passed since he was exposed to the gadolinium, the characteristic clinical features led to the suspicion of NSF. A biopsy of the right anterior tibial skin lesion was performed to examine tissue for disease. This revealed unique features of the NSF such as dermal thickening with collagen fibers extending into the subcutaneous septa and a large number of spindle-shaped fibrocytes (
Fig. 3). Neither eosinophilic tissue infiltration nor fat necrosis with calcification of adipocytes was found.
Fig. 3
Pathologic findings of the skin biopsy. (A) Thickened dermis with collagen deposits (H & E stain, × 40). (B) Collagen bundles in dermis with scant inflammatory cell infiltration and a well preserved adnexal structure (H & E stain, × 200). (C) The septal fibrosis of the subcutaneous fat, not accompanied by active inflammation or fat necrosis (H & E stain, × 200). (D) Infiltration of CD34 positive spindle cells.
H & E stain = hematoxylin and eosin stain.
Systemic methylprednisolone (20 mg/day) was administered for two weeks to treat the NSF. Additionally, UV-A1 topical phototherapy of 1.5 joule (J)/cm
2 was administered to the lower leg lesion twice a week. The patient underwent intensive physical therapy including stretching, pneumatic compression, and Transcutaneous Electric Nerve Stimulation . We administered MTX 15 mg weekly while reducing the methylprednisolone dose weekly by half until a dose of 2 mg/day was administered. Simultaneously, the UV-A1 intensity was gradually increased to 3.0 J/cm
2. After 6 months of treatment, the reddish edema improved and the skin induration decreased. Although the contracture of the ankles was still present (
Fig. 2), there was no further disease progression. Currently, the patient receives MTX (10 mg) weekly and methylprednisolone (2 mg) daily.
Ethics statement
Informed consent for publication of clinical data was submitted by the patient and the images are published with the consent of the patient.
DISCUSSION
NSF mostly occurs in patients with advanced CKD and end stage renal disease. These patients have a 1%–7% chance of developing NSF after exposure to GBCAs, especially type 1 GBCAs that contain linear ligands (such as gadodiamide, gadopentetate, and gadoversetamide). In comparison, type 2 GBCAs that contain macrocyclic ligands (such as gadoterate and gadoteridol) are less likely to be a risk factor for NSF. World-wide guidelines strongly restrict the usage of type 1 GBCAs in advanced CKD and in AKI; however, they do not regulate the types and dosages of GBCAs in patients with eGFR above 30 mL/min/1.73 m
2.
6 Only a few cases of NSF have been reported in patients with stage 3 CKD. These patients had risk factors like AKI on CKD state or coexisting proinflammatory event (major surgery, infection, malignancy, thrombosis or vascular event).
78
This is the first reported case of NSF occurring in a stage 3a CKD patient without previous known risk factor.
During the first visit, which was due to edema and redness of the lower extremities, we treated the patient for cellulitis. In the clinical setting, NSF could be easily misdiagnosed as cellulitis, panniculitis, or drug reaction. Additionally, the long latent period made it difficult to consider these manifestations as early markers of NSF. The period between GBCAs exposure and onset of NSF is approximately two to ten weeks (median, five weeks). However, our patient had been asymptomatic for about 26 weeks (185 days). This case suggests that when color change and edema occur in a CKD patient who underwent MRI, even if the MRI was performed a long time ago, early skin biopsy to detect NSF should be considered as a diagnostic option.
We investigated the possible causes of the NSF in our patient. First, the patient had a hypotrophic left kidney, which suggested that he could be more vulnerable to nephrotoxic stimuli. Second, although type 2 GBCAs was used, more than the recommended dose was administered. Gadoterate meglumine is one of ionic macrocycle-structured GBCAs which consist of the organic acid (tetraxetan) as a chelating agent and entirely excreted in renal pathway.
9 The recommended dose is 0.2 mL/kg (0.1 mmol/kg) body weight administered as an intravenous bolus injection. In this patient, 1.5-fold of adequate dose was used. Moreover, the consecutive usage of iodide contrast before gadolinium contrast might have been a burden to his renal function, because iodide contrast itself can cause free radical oxidative stress to renal parenchyme. Therefore, clinicians should be more cautious when using gadolinium contrast, even in patients with early CKD, and should carefully investigate any recent AKI event or individual risk factor. Also, weight adjusted proper GBCA dose and sufficient intervals should be applied when using radiologic contrasts in these patients.
There is no proven treatment for NSF to date. The restoration of renal function is crucial to prevent the progression of the disease. In some case reports, treatments including photopheresis, intravenous immunoglobulin, and local administration of interferon showed limited improvement of skin lesions.
1011 In our case, steroid and MTX in combination with UV-A therapy seemed to relieve the cutaneous symptoms. The pathophysiology of NSF is not fully known. One hypothesis is that gadolinium ion activates a vicious fibrotic cycle of dendritic cell maturation and transforming growth factor beta 1 (TGF-β1) production, which results in excessive collagen accumulation in the soft tissue. UV-A1 phototherapy decreases skin induration by inhibiting the production of TGF-β1 and procollagen.
12 MTX has been recommended as a treatment for early diffuse systemic sclerosis, especially for cutaneous symptoms. The effect of MTX on skin sclerosis is unclear; however, it is thought to decrease systemic immune and local inflammatory reactions and suppress the activities of profibrotic factors.
13
In conclusion, we described a patient who developed NSF during stage 3a CKD six months after first exposure to gadolinium. When using GBCAs in early stage CKD patients, clinicians should assess the risk factors for AKI and avoid exposure to multiple contrast agents on the same day. In addition, once NSF is suspected following long-term observation, prompt skin biopsy should be performed. In the absence of a definite therapeutic guideline, low dose MTX plus steroid combined with UA-1 phototherapy may be considered as a treatment option for NSF.







 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download