1. Kozinszky Z, Sikovanyecz J, Pásztor N. Severe midtrimester oligohydramnios: treatment strategies. Curr Opin Obstet Gynecol. 2014; 26(2):67–76. PMID:
24614021.
2. Klaassen I, Neuhaus TJ, Mueller-Wiefel DE, Kemper MJ. Antenatal oligohydramnios of renal origin: long-term outcome. Nephrol Dial Transplant. 2007; 22(2):432–439. PMID:
17065192.

3. Gribouval O, Gonzales M, Neuhaus T, Aziza J, Bieth E, Laurent N, et al. Mutations in genes in the renin-angiotensin system are associated with autosomal recessive renal tubular dysgenesis. Nat Genet. 2005; 37(9):964–968. PMID:
16116425.

4. Gubler MC, Antignac C. Renin-angiotensin system in kidney development: renal tubular dysgenesis. Kidney Int. 2010; 77(5):400–406. PMID:
19924102.

5. Plazanet C, Arrondel C, Chavant F, Gubler MC. Fetal renin-angiotensin-system blockade syndrome: renal lesions. Pediatr Nephrol. 2014; 29(7):1221–1230. PMID:
24477978.

6. Vivante A, Kohl S, Hwang DY, Dworschak GC, Hildebrandt F. Single-gene causes of congenital anomalies of the kidney and urinary tract (CAKUT) in humans. Pediatr Nephrol. 2014; 29(4):695–704. PMID:
24398540.

7. Lee KH, Gee HY, Shin JI. Genetics of vesicoureteral reflux and congenital anomalies of the kidney and urinary tract. Investig Clin Urol. 2017; 58(Suppl 1):S4–S13.

8. Yosypiv IV. Renin-angiotensin system in ureteric bud branching morphogenesis: implications for kidney disease. Pediatr Nephrol. 2014; 29(4):609–620. PMID:
24061643.

9. Lacoste M, Cai Y, Guicharnaud L, Mounier F, Dumez Y, Bouvier R, et al. Renal tubular dysgenesis, a not uncommon autosomal recessive disorder leading to oligohydramnios: role of the Renin-Angiotensin system. J Am Soc Nephrol. 2006; 17(8):2253–2263. PMID:
16790508.

10. Gubler MC. Renal tubular dysgenesis. Pediatr Nephrol. 2014; 29(1):51–59. PMID:
23636579.

11. Fiselier TJ, Lijnen P, Monnens L, van Munster P, Jansen M, Peer P. Levels of renin, angiotensin I and II, angiotensin-converting enzyme and aldosterone in infancy and childhood. Eur J Pediatr. 1983; 141(1):3–7. PMID:
6315441.

12. Gribouval O, Morinière V, Pawtowski A, Arrondel C, Sallinen SL, Saloranta C, et al. Spectrum of mutations in the renin-angiotensin system genes in autosomal recessive renal tubular dysgenesis. Hum Mutat. 2012; 33(2):316–326. PMID:
22095942.

13. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015; 17(5):405–424. PMID:
25741868.

14. Ruf K, Wirbelauer J, Beissert A, Frieauff E. Successful treatment of severe arterial hypotension and anuria in a preterm infant with renal tubular dysgenesis- a case report. Matern Health Neonatol Perinatol. 2018; 4(1):27. PMID:
30598831.

15. Miyawaki M, Okutani T, Higuchi R, Yoshikawa N. Plasma angiotensin II concentrations in the early neonatal period. Arch Dis Child Fetal Neonatal Ed. 2006; 91(5):F359–62. PMID:
16595591.

16. Bergmann C. ARPKD and early manifestations of ADPKD: the original polycystic kidney disease and phenocopies. Pediatr Nephrol. 2015; 30(1):15–30. PMID:
24584572.

17. Wiesel A, Queisser-Luft A, Clementi M, Bianca S, Stoll C, Group ES, et al. Prenatal detection of congenital renal malformations by fetal ultrasonographic examination: an analysis of 709,030 births in 12 European countries. Eur J Med Genet. 2005; 48(2):131–144. PMID:
16053904.

18. Richer J, Daoud H, Geier P, Jarinova O, Carson N, Feberova J, et al. Resolution of refractory hypotension and anuria in a premature newborn with loss-of-function of ACE. Am J Med Genet A. 2015; 167(7):1654–1658. PMID:
25899979.

19. Kim SY, Kang HG, Kim EK, Choi JH, Choi Y, Cheong HI. Survival over 2 years of autosomal-recessive renal tubular dysgenesis. Clin Kidney J. 2012; 5(1):56–58. PMID:
26069751.

20. Fila M, Morinière V, Eckart P, Terzic J, Gubler MC, Antignac C, et al. Bi-allelic mutations in renin-angiotensin system genes, associated with renal tubular dysgenesis, can also present as a progressive chronic kidney disease. Pediatr Nephrol. 2020; 35(6):1125–1128. PMID:
32198635.

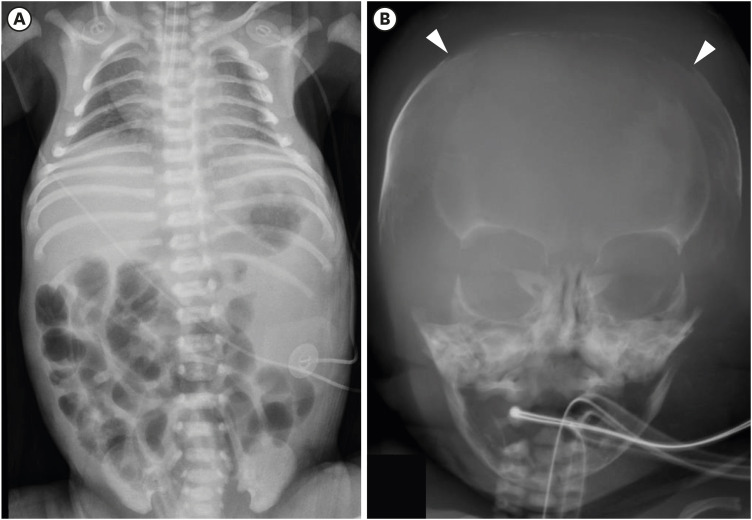
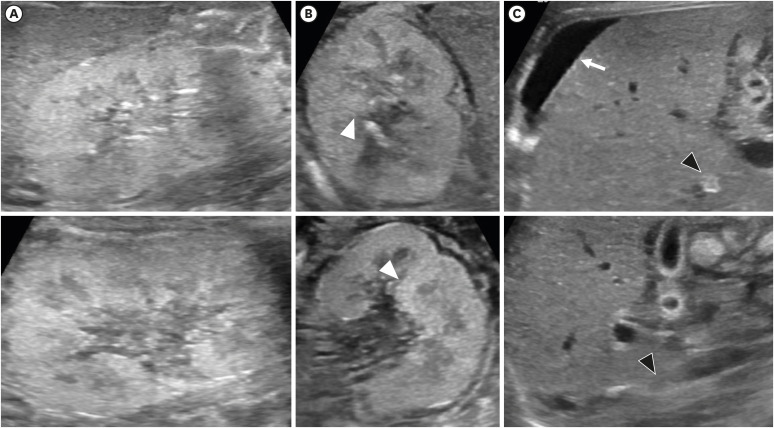
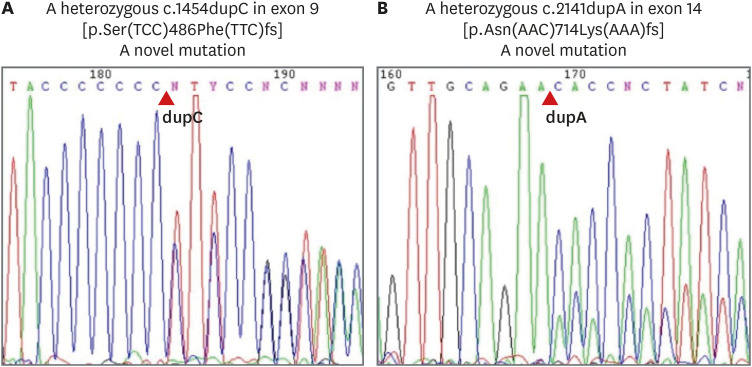
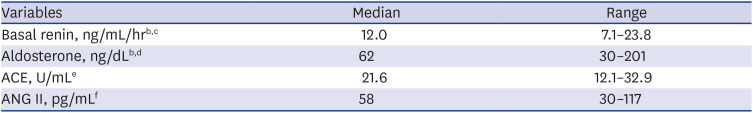




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download