1. Lin S, Liu X, Le LH, Hwang SA. Chronic exposure to ambient ozone and asthma hospital admissions among children. Environ Health Perspect. 2008; 116(12):1725–1730. PMID:
19079727.

2. Galán I, Tobías A, Banegas JR, Aránguez E. Short-term effects of air pollution on daily asthma emergency room admissions. Eur Respir J. 2003; 22(5):802–808. PMID:
14621088.

3. Medina-Ramón M, Zanobetti A, Schwartz J. The effect of ozone and PM10 on hospital admissions for pneumonia and chronic obstructive pulmonary disease: a national multicity study. Am J Epidemiol. 2006; 163(6):579–588. PMID:
16443803.

4. Ramadour M, Burel C, Lanteaume A, Vervloet D, Charpin D, Brisse F, et al. Prevalence of asthma and rhinitis in relation to long-term exposure to gaseous air pollutants. Allergy. 2000; 55(12):1163–1169. PMID:
11117274.

5. Wang T, Cheung VT, Anson M, Li YS. Ozone and related gaseous pollutants in the boundary layer of eastern China: overview of the recent measurements at a rural site. Geophys Res Lett. 2001; 28(12):2373–2376.

6. Jang AS, Choi IS, Yang SY, Kim YG, Lee JH, Park SW, et al. Antioxidant responsiveness in BALB/c mice exposed to ozone. Respiration. 2005; 72(1):79–84. PMID:
15753639.

7. Jang AS, Choi IS, Lee JH, Park CS, Park CS. Prolonged ozone exposure in an allergic airway disease model: adaptation of airway responsiveness and airway remodeling. Respir Res. 2006; 7(1):24. PMID:
16472404.

8. Jang AS, Choi IS, Takizawa H, Rhim T, Lee JH, Park SW, et al. Additive effect of diesel exhaust particulates and ozone on airway hyperresponsiveness and inflammation in a mouse model of asthma. J Korean Med Sci. 2005; 20(5):759–763. PMID:
16224148.

9. Larsen ST, Matsubara S, McConville G, Poulsen SS, Gelfand EW. Ozone increases airway hyperreactivity and mucus hyperproduction in mice previously exposed to allergen. J Toxicol Environ Health A. 2010; 73(11):738–747. PMID:
20391116.

10. Harijith A, Ebenezer DL, Natarajan V. Reactive oxygen species at the crossroads of inflammasome and inflammation. Front Physiol. 2014; 5:352. PMID:
25324778.

11. Girotti AW. Mechanisms of lipid peroxidation. J Free Radic Biol Med. 1985; 1(2):87–95. PMID:
3915303.

12. Kelly FJ, Mudway IS. Protein oxidation at the air-lung interface. Amino Acids. 2003; 25(3-4):375–396. PMID:
14661098.

13. Vasu VT, Oommen S, Lim Y, Valacchi G, Hobson B, Eirserich JP, et al. Modulation of ozone-sensitive genes in alpha-tocopherol transfer protein null mice. Inhal Toxicol. 2010; 22(1):1–16.

14. Qu F, Qin XQ, Cui YR, Xiang Y, Tan YR, Liu HJ, et al. Ozone stress down-regulates the expression of cystic fibrosis transmembrane conductance regulator in human bronchial epithelial cells. Chem Biol Interact. 2009; 179(2-3):219–226. PMID:
19061877.

15. Matsui H, Grubb BR, Tarran R, Randell SH, Gatzy JT, Davis CW, et al. Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell. 1998; 95(7):1005–1015. PMID:
9875854.

16. Coakley RD, Grubb BR, Paradiso AM, Gatzy JT, Johnson LG, Kreda SM, et al. Abnormal surface liquid pH regulation by cultured cystic fibrosis bronchial epithelium. Proc Natl Acad Sci U S A. 2003; 100(26):16083–16088. PMID:
14668433.

17. Pedemonte N, Caci E, Sondo E, Caputo A, Rhoden K, Pfeffer U, et al. Thiocyanate transport in resting and IL-4-stimulated human bronchial epithelial cells: role of pendrin and anion channels. J Immunol. 2007; 178(8):5144–5153. PMID:
17404297.

18. Nakagami Y, Favoreto S Jr, Zhen G, Park SW, Nguyenvu LT, Kuperman DA, et al. The epithelial anion transporter pendrin is induced by allergy and rhinovirus infection, regulates airway surface liquid, and increases airway reactivity and inflammation in an asthma model. J Immunol. 2008; 181(3):2203–2210. PMID:
18641360.

19. Lee HJ, Yoo JE, Namkung W, Cho HJ, Kim K, Kang JW, et al. Thick airway surface liquid volume and weak mucin expression in pendrin-deficient human airway epithelia. Physiol Rep. 2015; 3(8):e12480. PMID:
26243215.

20. Kuperman DA, Lewis CC, Woodruff PG, Rodriguez MW, Yang YH, Dolganov GM, et al. Dissecting asthma using focused transgenic modeling and functional genomics. J Allergy Clin Immunol. 2005; 116(2):305–311. PMID:
16083784.

21. Lewis CC, Yang JY, Huang X, Banerjee SK, Blackburn MR, Baluk P, et al. Disease-specific gene expression profiling in multiple models of lung disease. Am J Respir Crit Care Med. 2008; 177(4):376–387. PMID:
18029791.

22. Ishida A, Ohta N, Suzuki Y, Kakehata S, Okubo K, Ikeda H, et al. Expression of pendrin and periostin in allergic rhinitis and chronic rhinosinusitis. Allergol Int. 2012; 61(4):589–595. PMID:
22918213.
23. Nakao I, Kanaji S, Ohta S, Matsushita H, Arima K, Yuyama N, et al. Identification of pendrin as a common mediator for mucus production in bronchial asthma and chronic obstructive pulmonary disease. J Immunol. 2008; 180(9):6262–6269. PMID:
18424749.
24. Xu Y, Szép S, Lu Z. The antioxidant role of thiocyanate in the pathogenesis of cystic fibrosis and other inflammation-related diseases. Proc Natl Acad Sci U S A. 2009; 106(48):20515–20519. PMID:
19918082.

25. Wagner CA, Finberg KE, Stehberger PA, Lifton RP, Giebisch GH, Aronson PS, et al. Regulation of the expression of the Cl
−/anion exchanger pendrin in mouse kidney by acid-base status. Kidney Int. 2002; 62(6):2109–2117. PMID:
12427135.
26. Frische S, Kwon TH, Frøkiaer J, Madsen KM, Nielsen S. Regulated expression of pendrin in rat kidney in response to chronic NH
4Cl or NaHCO
3 loading. Am J Physiol Renal Physiol. 2003; 284(3):F584–F593. PMID:
12556366.
27. Amlal H, Petrovic S, Xu J, Wang Z, Sun X, Barone S, et al. Deletion of the anion exchanger Slc26a4 (pendrin) decreases apical Cl
−/HCO
3− exchanger activity and impairs bicarbonate secretion in kidney collecting duct. Am J Physiol Cell Physiol. 2010; 299(1):C33–41. PMID:
20375274.
28. Hafner P, Grimaldi R, Capuano P, Capasso G, Wagner CA. Pendrin in the mouse kidney is primarily regulated by Cl
− excretion but also by systemic metabolic acidosis. Am J Physiol Cell Physiol. 2008; 295(6):C1658–67. PMID:
18971389.
29. Kim YH, Verlander JW, Matthews SW, Kurtz I, Shin W, Weiner ID, et al. Intercalated cell H
+/OH
− transporter expression is reduced in Slc26a4 null mice. Am J Physiol Renal Physiol. 2005; 289(6):F1262–72. PMID:
16144965.
30. Neuhaus-Steinmetz U, Glaab T, Daser A, Braun A, Lommatzsch M, Herz U, et al. Sequential development of airway hyperresponsiveness and acute airway obstruction in a mouse model of allergic inflammation. Int Arch Allergy Immunol. 2000; 121(1):57–67. PMID:
10686510.

31. McGovern TK, Robichaud A, Fereydoonzad L, Schuessler TF, Martin JG. Evaluation of respiratory system mechanics in mice using the forced oscillation technique. J Vis Exp. 2013; (75):e50172. PMID:
23711876.

32. Choi JM, Ahn MH, Chae WJ, Jung YG, Park JC, Song HM, et al. Intranasal delivery of the cytoplasmic domain of CTLA-4 using a novel protein transduction domain prevents allergic inflammation. Nat Med. 2006; 12(5):574–579. PMID:
16604087.

33. Bonner JC, Rice AB, Moomaw CR, Morgan DL. Airway fibrosis in rats induced by vanadium pentoxide. Am J Physiol Lung Cell Mol Physiol. 2000; 278(1):L209 –16. PMID:
10645909.

34. Lee JU, Cheong HS, Shim EY, Bae DJ, Chang HS, Uh ST, et al. Gene profile of fibroblasts identify relation of CCL8 with idiopathic pulmonary fibrosis. Respir Res. 2017; 18(1):3. PMID:
28057004.

35. Gould NS, Gauthier S, Kariya CT, Min E, Huang J, Brian DJ. Hypertonic saline increases lung epithelial lining fluid glutathione and thiocyanate: two protective CFTR-dependent thiols against oxidative injury. Respir Res. 2010; 11(1):119. PMID:
20799947.

36. Gorrieri G, Scudieri P, Caci E, Schiavon M, Tomati V, Sirci F, et al. Goblet cell hyperplasia requires high bicarbonate transport to support mucin release. Sci Rep. 2016; 6(1):36016. PMID:
27786259.

37. Kuperman DA, Huang X, Nguyenvu L, Hölscher C, Brombacher F, Erle DJ. IL-4 receptor signaling in Clara cells is required for allergen-induced mucus production. J Immunol. 2005; 175(6):3746–3752. PMID:
16148120.

38. Kuperman DA, Huang X, Koth LL, Chang GH, Dolganov GM, Zhu Z, et al. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat Med. 2002; 8(8):885–889. PMID:
12091879.

39. Lee H, Kim EK, Kim HY, Kim TI. Effects of exposure to ozone on the ocular surface in an experimental model of allergic conjunctivitis. PLoS One. 2017; 12(1):e0169209. PMID:
28046113.

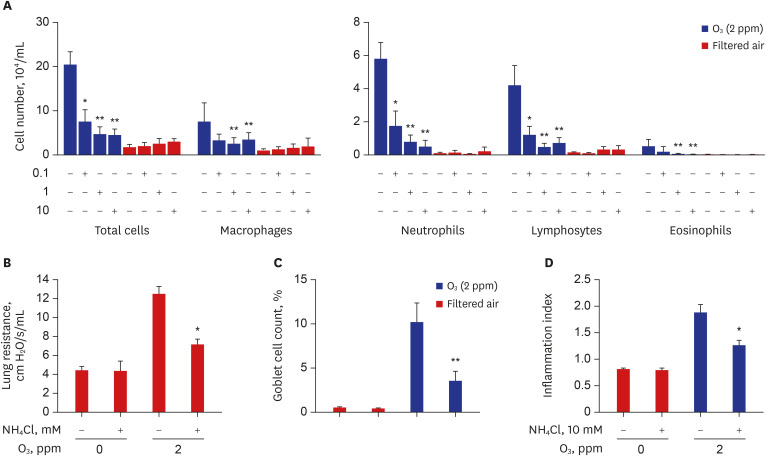
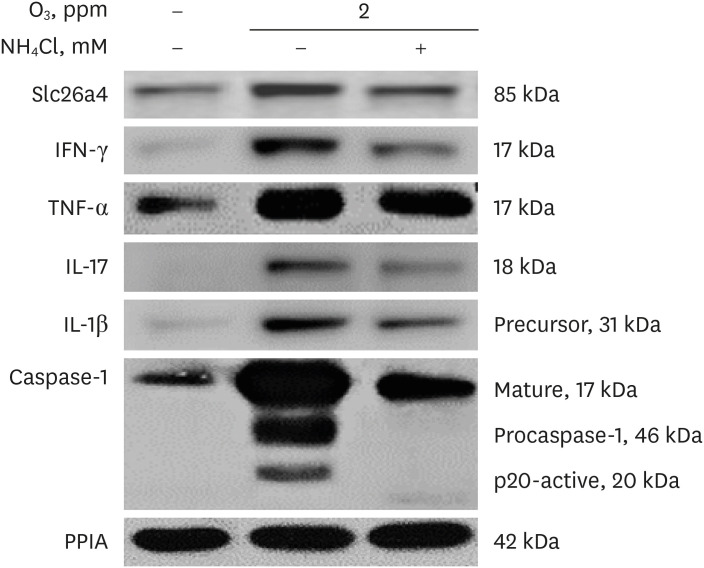
40. Che L, Jin Y, Zhang C, Lai T, Zhou H, Xia L, et al. Ozone-induced IL-17A and neutrophilic airway inflammation is orchestrated by the caspase-1-IL-1 cascade. Sci Rep. 2016; 6(1):18680. PMID:
26739627.

41. Ryan LK, Copeland LR, Daniels MJ, Costa ER, Selgrade MJ. Proinflammatory and Th1 cytokine alterations following ultraviolet radiation enhancement of disease due to influenza infection in mice. Toxicol Sci. 2002; 67(1):88–97. PMID:
11961220.








 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download