This article has been
cited by other articles in ScienceCentral.
Abstract
A 60-year-old male patient with coronavirus disease-2019 showed new onset ST-segment elevation in V1–V2 leads on electrocardiogram and cardiac enzyme elevation in intensive care unit. He had a history of type 2 diabetes mellitus, hypertension, and dyslipidemia. He was receiving mechanical ventilation and veno-venous extracorporeal membrane oxygenation treatment for severe hypoxia. Two-D echocardiogram showed regional wall motion abnormalities. We performed primary percutaneous coronary intervention for acute myocardial infarction complicating cardiogenic shock under hemodynamic support. He expired on the 16th day of admission because of cardiogenic shock and multi-organ failure. Active surveillance and intensive treatment strategy are important for saving lives of COVID-19 patients with acute myocardial infarction.
Go to :

Graphical Abstract
Go to :

Keywords: SARS-CoV-2, COVID-19, Acute Myocardial Infarction, Cardiogenic Shock
INTRODUCTION
Since the first report of coronavirus disease 2019 (COVID-19) in Hubei Province, China in December 2019, it has been spreading rapidly worldwide, and the World Health Organization declared a pandemic on March 11, 2020. In Daegu, Korea, there was rapid increase in COVID-19 patients following the first outbreak on February 18, 2020. The pandemic of COVID-19 acted as a significant burden on the medical capacity of local medical institutions, and local medical institutions concentrated a large number of personnel and facilities to prevent and treat COVID-19 infection. Therefore, in such a pandemic, it is very difficult to properly deal with emergency situation such as acute myocardial infarction (AMI). We herein present our first case of percutaneous coronary intervention (PCI) for patients with COVID-19 infection who developed AMI with cardiogenic shock during hospitalization in intensive care unit.
Go to :

CASE DESCRIPTION
A 60-years-old male visited the emergency room for general weakness and poor oral intake for 10 days on March 18, 2020. He had a history of type 2 diabetes mellitus, hypertension for 20 years, and dyslipidemia for 10 years. Initially, he did not complain of respiratory symptoms such as cough, sputum, dyspnea, or febrile chilling sensation. His initial vital signs were pertinent for a blood pressure of 160/100 mmHg, a heart rate of 90 beats/min, a body temperature 36.8°C, a respiratory rate of 20 breaths/min, and peripheral O
2 saturation of 94% on room air. However, the chest X-ray demonstrated bilateral patchy infiltration (
Fig. 1). He had a history of close contact with a COVID-19 patient during medical practice as a physician on February 25, 2020. Accordingly, a real-time reverse transcriptase polymerase chain reaction test for COVID-19 was done and confirmed COVID-19 positive on March 19, 2020. Although an anti-viral agent (lopinavir/ritonavir) and antibiotics were administered immediate after admission, his condition continued to deteriorate during hospitalization. Intubation and mechanical ventilation was applied on the 5th day of admission. Continuous renal replacement therapy was started on the 6th day of admission and veno-venous extracorporeal membrane oxygenation (V-V ECMO) was applied on the 13th day of admission. On the 14th day of hospitalization, his vital signs became unstable. Chest X-ray revealed peribronchial ground glass opacities and nodular opacities in both lung fields (
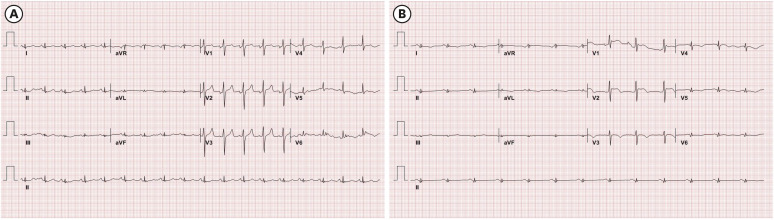
Fig. 1). On the electrocardiogram (ECG), ST elevation in V1–V2 leads and T wave inversion in V3–V6 leads were newly observed (
Fig. 2). Laboratory testing demonstrated elevated cardiac troponin I of 46.1 ng/mL (reference range ≤ 0.045 ng/mL) and N-terminal pro-B-type natriuretic peptide of 1,971.5 pg/mL (reference range ≤ 125.0 pg/mL). Transthoracic two-D echocardiogram (TTE) revealed severe decreased left ventricular ejection fraction of 30%, and regional wall motion abnormalities at apex and apical septal segments.
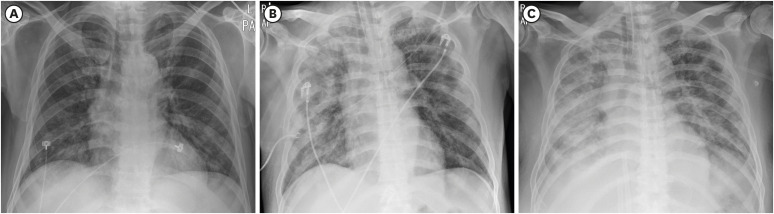 | Fig. 1 Chest X-ray images. Chest X-ray on the day of admission (A), day 6 (B), day 14 (C) of hospitalization. Peribronchial ground glass opacities and nodular opacities in both lung fields were deteriorated.
|
 | Fig. 2
Interval changes of ECG. ECG on the day of admission (A) and day 14 (B) of hospitalization. New onset ST-segment elevation in V1–V2 leads and T wave inversion in V3–V6 leads were observed.
aVR = augmented vector right, aVL = augmented vector left; aVF = augmented vector foot, ECG = electrocardiogram.

|
Despite the use of inotropes and V-V ECMO, blood pressure and peripheral O
2 saturation gradually decreased. Therefore, we decided to perform urgent coronary angiography (CAG). A negative pressure isolation chamber was used to minimize possible infection in the patient transfer process. A minimum number of staffs including three cardiologists, a nurse, and an X-ray technician was allowed to participate in procedure. Before the patient entered the catheterization room, medical personnel entered the room first wearing level D personal protective equipment including powered air purifying respirators (
Fig. 3). Baseline CAG revealed multivessel disease. There was 80% diameter stenosis in the mid and distal portion of the left anterior descending artery (LAD), 90% diameter stenosis in the proximal ramus intermedius coronary artery (pRI), 90% diameter stenosis in the proximal left circumflex artery (pLCX), and 95% diameter stenosis in the proximal and distal right coronary artery (RCA) (
Fig. 4). It was difficult to distinguish the culprit lesion by ECG, TTE, and CAG. We decided to perform complete revascularization for multivessel coronary lesions because his vital signs were unstable. First, pre-balloon angioplasty using a 2.5 × 20 mm balloon was done and drug eluting stent (3.5 × 32 mm) was implanted in the proximal RCA lesion. Second, plain old balloon angioplasty (POBA) using a 2.5 × 20 mm balloon on the pLCX lesion was done. Third, POBA using a 2.5 × 20 mm balloon was done at the pRI lesion. Fourth, pre-balloon angioplasty using a 2.5 × 20 mm balloon was done and a drug eluting stent (3.0 × 28 mm) was implanted in the mid LAD lesion. Finally, we obtained Thrombolysis In Myocardial Infarction (TIMI) grade 3 flow in LAD, LCX, and RI, separately, and TIMI grade 2 flow in RCA (
Fig. 5). The total amount of contrast media used was 150 cc and total procedural time was 72 minutes. There were no specific complications during the procedure.
 | Fig. 3 Scene at the time of procedure. Medical staffs wore level D personal protective equipment including 2 pairs of surgical gloves, N95 mask, goggle, face shield, protective suit, leg covers, and sterile surgical gown.
|
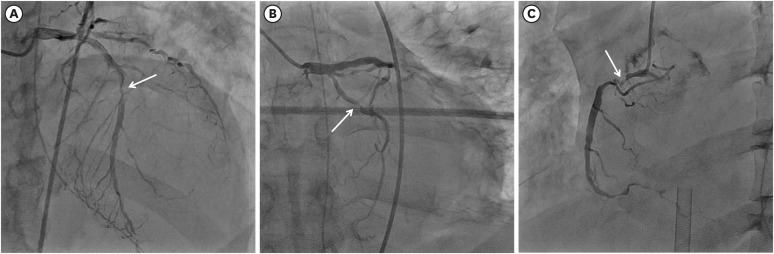 | Fig. 4 Baseline coronary angiogram. Coronary angiogram showed severe stenosis in mid portion of left descending coronary artery (A), proximal portion of left circumflex coronary artery (B), and proximal portion of right coronary artery (C). White arrows indicate coronary lesions in each vessel.
|
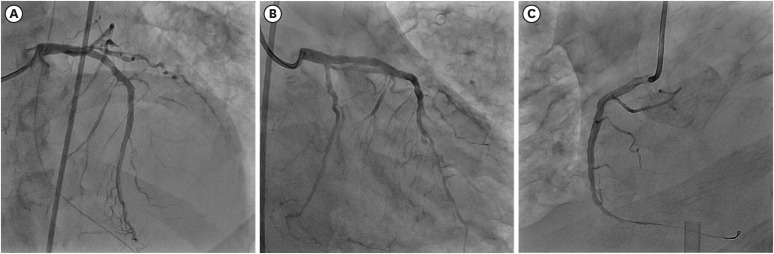 | Fig. 5 Final coronary angiogram after percutaneous coronary intervention. (A) Drug eluting stents were implanted in mid portion of left descending coronary artery and (C) proximal portion of right coronary artery. (B) Balloon angioplasty was done in proximal portion of left circumflex coronary artery.
|
Immediately after PCI, V-V ECMO was converted to veno-arterial ECMO for hemodynamic support. Despite maximum medical therapy, the patient's condition gradually deteriorated and he did not recover from the cardiogenic shock. One day after the PCI, an intra-aortic balloon pump was applied in anticipation of improvement of cardiogenic shock. However, unfortunately, he expired the next day (16th day of admission) because of cardiogenic shock and multi-organ failure.
Ethics statement
The study protocol was reviewed and approved by the Institutional Review Board of School of Medicine, Kyungpook National University (IRB No. 2020-06-034).
Go to :

DISCUSSION
Since the first confirmed cases of COVID-19 occurred on February 18, 2020, in Daegu, Korea, the number of confirmed cases increased sharply up to 6,704 cases, and 115 deaths occurred by April 1, 2020. This is the first case of a patient with COVID-19 who underwent PCI for AMI complicating cardiogenic shock. In our case, there are two noteworthy lessons regarding the COVID-19 pandemic.
First, there are significant associations between cardiovascular risk factors and mortality in COVID-19. Cardiovascular comorbidities are common in patients with COVID-19 infection. However, in previous studies, relative frequency of cardiovascular risk factors or underlying cardiovascular conditions in available COVID-19 cohorts was quite variable.
1 Hypertension was presented from 6.5% to 38.6% and diabetes mellitus presented from 7.3% to 19.5% of confirmed cases. The prevalence of cardiovascular disease in COVID-19 patients was also variable from 4.0% to 14.6%. Therefore, it remains uncertain whether hypertension, diabetes mellitus, and cardiovascular disease are causally linked with COVID-19 infection. However, the patients who develop severe disease are more likely to be vulnerable because of comorbid disease such as hypertension, diabetes mellitus, and cardiovascular disease. COVID-19 mainly affects the respiratory system, including the lung, but affects multiple organs, especially the cardiovascular system.
2,3 In previous reports, cases of pericarditis,
4 myocarditis,
5 stress induced cardiomyopathy,
6,7 and arrhythmia occurred in patients with COVID-19,
8 and cases of pulmonary thromboembolism,
9 coronary artery thrombosis,
10 and STEMI were also reported.
11,12 However, studies on how COVID-19 affect AMI is limited. The patient suffered from diabetes mellitus and was suspected of diabetic ketoacidosis with serum glucose of 655 mg/dL, hemoglobin A1C of 11.4%, and ketone body of 4.9 mmol/L (reference range ≤ 0.6 mmol/L) at the time of admission.
13 In addition, he had a high risk of AMI due to severe respiratory failure and renal failure during hospitalization. Although it is not understood how COVID-19 affects development of AMI, severe respiratory failure and multi-organ failure may directly or indirectly affect the development of AMI.
14 In particular, the risk of AMI may increase in patients with underlying conditions such as old age, hypertension, diabetes mellitus, dyslipidemia, and chronic kidney disease. Therefore, it is necessary to closely observe and properly test ECG, cardiac enzymes, and TTE (if suspected) in patients with cardiovascular risk factors or underlying cardiovascular conditions during the COVID-19 pandemic.
Second, significant time delay between diagnosis and procedure may occur in COVID-19 patients. Reduction of reperfusion time is crucial for system of care in patients with AMI. This treatment principle of AMI is not significantly different even in COVID-19 confirmed patients.
15,16 However, in real-world practice, it is possible to have a significant time delay between AMI diagnosis and the actual procedure because the primary PCI of a COVID-19 patient is accompanied by the possibility of COVID-19 transmission in hospital facilities, medical staff, and other patients. Therefore, there must be a policy for patient transportation and facility isolation in advance. In addition, there must be a standard sterilization processes for the facility after the procedure. First of all, the most important thing is that sufficient personal protection equipment for all medical staff are provided during the whole procedure process. In our case, although he had AMI with cardiogenic shock, primary PCI was performed at the end of the scheduled procedures to minimize exposure of other patients. Therefore, several hours of time delay occurred between the PCI decision and actual PCI implementation. We should make an effort to reduce transportation and personal protection-related time delay in COVID-19 patients.
Finally, when AMI with cardiogenic shock is accompanied by COVID-19, a patient's recovery is very difficult. Therefore, we should remember that active surveillance and intensive treatment strategy without time delay are important for saving lives even in COVID-19 patients with AMI.
Go to :










 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download