1. Naicker S, Yang CW, Hwang SJ, Liu BC, Chen JH, Jha V. The novel coronavirus 2019 epidemic and kidneys. Kidney Int. 2020; 97(5):824–828. PMID:
32204907.

2. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020; 395(10223):497–506. PMID:
31986264.

3. Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020; 382(13):1199–1207. PMID:
31995857.
4. Phan LT, Nguyen TV, Luong QC, Nguyen TV, Nguyen HT, Le HQ, et al. Importation and human-to-human transmission of a novel coronavirus in Vietnam. N Engl J Med. 2020; 382(9):872–874. PMID:
31991079.

5. Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020; 382(10):929–936. PMID:
32004427.

6. Giovanetti M, Benvenuto D, Angeletti S, Ciccozzi M. The first two cases of 2019-nCoV in Italy: where they come from? J Med Virol. 2020; 92(5):518–521. PMID:
32022275.

9. Ministry of Health & Welfare (KR). COVID-19. Updated 2020. Accessed April 25, 2020.
http://ncov.mohw.go.kr.
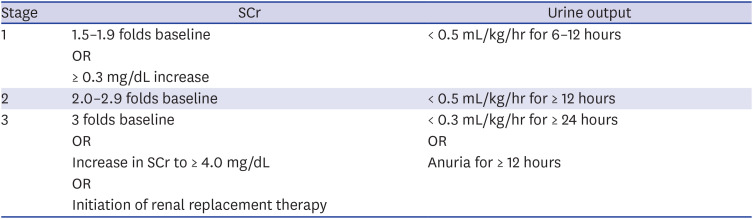
11. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012; 2(1):1–138.
12. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013; 3(1):1–150.
13. Davis R, Jones JS, Barocas DA, Castle EP, Lang EK, Leveillee RJ, et al. Diagnosis, evaluation and follow-up of asymptomatic microhematuria (AMH) in adults: AUA guideline. J Urol. 2012; 188(6):Suppl. 2473–2481. PMID:
23098784.

14. Wei PF. Diagnosis and treatment protocol for novel coronavirus pneumonia (trial version 7). Chin Med J (Engl). 2020; 133(9):1087–1095. PMID:
32358325.
15. Xu X, Chen P, Wang J, Feng J, Zhou H, Li X, et al. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci. 2020; 63(3):457–460. PMID:
32009228.

16. Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020; 579(7798):270–273. PMID:
32015507.
17. Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020; 14(2):185–192. PMID:
32170560.

18. Chu KH, Tsang WK, Tang CS, Lam MF, Lai FM, To KF, et al. Acute renal impairment in coronavirus-associated severe acute respiratory syndrome. Kidney Int. 2005; 67(2):698–705. PMID:
15673319.

19. Cha RH, Joh JS, Jeong I, Lee JY, Shin HS, Kim G, et al. Renal complications and their prognosis in Korean patients with Middle East respiratory syndrome-coronavirus from the central MERS-CoV designated hospital. J Korean Med Sci. 2015; 30(12):1807–1814. PMID:
26713056.

20. Wang L, Li X, Chen H, Yan S, Li D, Li Y, et al. Coronavirus disease 19 infection does not result in acute kidney injury: an analysis of 116 hospitalized patients from Wuhan, China. Am J Nephrol. 2020; 51(5):343–348. PMID:
32229732.

21. Hu Y, Sun J, Dai Z, Deng H, Li X, Huang Q, et al. Prevalence and severity of corona virus disease 2019 (COVID-19): a systematic review and meta-analysis. J Clin Virol. 2020; 127:104371. PMID:
32315817.

22. Birn H, Christensen EI. Renal albumin absorption in physiology and pathology. Kidney Int. 2006; 69(3):440–449. PMID:
16514429.

23. D'Amico G, Bazzi C. Pathophysiology of proteinuria. Kidney Int. 2003; 63(3):809–825. PMID:
12631062.
24. Vinclair C, De Montmollin E, Sonneville R, Reuter J, Lebut J, Cally R, et al. Factors associated with major adverse kidney events in patients who underwent veno-arterial extracorporeal membrane oxygenation. Ann Intensive Care. 2020; 10(1):44. PMID:
32307616.

25. Hsu CK, Wu IW, Chen YT, Tsai TY, Tsai FC, Fang JT, et al. Acute kidney disease stage predicts outcome of patients on extracorporeal membrane oxygenation support. PLoS One. 2020; 15(4):e0231505. PMID:
32268348.

26. Sinha Ray A, Haikal A, Hammoud KA, Yu AS. Vancomycin and the risk of AKI: a systematic review and meta-analysis. Clin J Am Soc Nephrol. 2016; 11(12):2132–2140. PMID:
27895134.

27. Deurdulian C, Tchelepi H. Imaging-based monitoring of the renal graft. In : Orlando G, Remuzzi G, Williams DF, editors. Kidney Transplantation, Bioengineering, and Regeneration. London: Academic Press;2017. p. 373–402.
28. Cereda M, Neligan P, Shashaty MGS, Horak J. Renal diseases. In : Fleisher LA, editor. Anesthesia and Uncommon Disease. 6th ed. Philadelphia, PA: Saunders;2012. p. 225–250.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download