Abstract
Coronavirus disease 2019 (COVID-19) is rapidly spreading around the world, causing much morbidity and mortality everywhere. However, effective treatments or vaccines are still not available. Although convalescent plasma (CP) therapy can be useful in the treatment of COVID-19, it has not been widely used in Korea because of the concerns about adverse effects and the difficulty in matching patients to donors. The use of ABO-incompatible plasma is not contraindicated in treatment, but can be hesitated due to the lack of experience of physicians. Here, we describe a 68-year old man with COVID-19 who was treated ABO-incompatible plasma therapy; additionally, we comment on the acute side effects associated with ABO mismatch transfusion. To overcome the obstacles of donor-recipient connections (schedule and distance), we propose the storage of frozen plasma, modification of the current Blood Management Law, and the establishment of a CP bank. We suggest that experience gained in CP therapy will be useful for not only the treatment of COVID-19, but also for coping with new emerging infectious diseases.
Go to : 
Graphical Abstract
Go to : 
Coronavirus disease 2019 (COVID-19), a disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was first detected in December 2019 in Wuhan, Hubei Province, China.1 SARS-CoV-2 is a member of the coronavirus family, which includes the SARS-CoV and the Middle East respiratory syndrome coronavirus (MERS-CoV), both of which caused outbreaks in 2003 and 2015, respectively. COVID-19 is mainly transmitted via respiratory droplets and has been widely and rapidly spreading to other countries outside mainland China since January 2020. As of 19 June 2020, more than 455,000 deaths have been recorded worldwide, and the number of confirmed patients and deaths has been rising.
Several drugs have been administered in an attempt to treat COVID-19, but no treatment guidelines have been established thus far. Drugs such as lopinavir/ritonavir2 and hydroxychloroquine3 have been used since the emergence of the disease without proven benefits. Remdesivir has recently been identified as a promising treatment candidate and has been reported to lower hospitalization and mortality rates.4 However, large-scale clinical studies are needed to establish its efficacy and safety.5 Although many attempts have been made to reposition drugs that can delay the replication or the entry of SARS-CoV-2 into the cell, or for immune modulation, their effects are still difficult to predict.67 In addition, considering the reports on the genetic variations of SARS-CoV-2,8 it is difficult to predict when a vaccine will become available. Therapeutic effects of convalescent plasma (CP) have been reported in various respiratory viral infections.9 As no effective treatment is currently available, CP has also been used for the treatment of COVID-19.101112 However, due to various barriers, CP is not yet widely used in Korea. Here, we describe a case report with CP therapy, and comment on the obstacles in the use of plasma therapy.
Go to : 
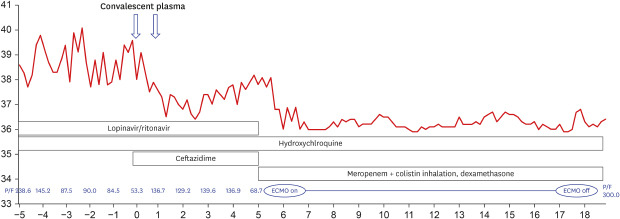
On March 27, 2020, a 68-year-old man came to our hospital for fever that occurred 7 days before admission. He was diagnosed with COVID-19 infection by polymerase chain reaction (AllplexTM 2019-nCoV Assay™; Seegene Co., Seoul, Korea) cycle threshold (CT) value of E gene: 20.1, RdRp: 20.8l, and N: 22.83 from nasopharynx), and although pneumonia could not be detected in the chest roentgenogram (CXR), his body temperature rose to 40°C. From the first day of hospitalization, he received hydroxychloroquine (200 mg every 12 hours) and lopinavir/ritonavir (400/100 mg every 12 hours); pneumonia was detected in his CXR on the third day of hospitalization. His respiratory distress progressed gradually, and a high-flow nasal canula was applied on the 5th day of hospitalization. On the 9th day of hospitalization, his pneumonia had progressed (E gene: 27.71, RdRp: 29.17, and N: 29.98 from nasopharynx; RdRP: 37.08 and N: 35.62 from sputum), and his PaO2/FiO2 ratio had deteriorated to 53. CP transfusion treatment was conducted with mechanical ventilation. His ABO blood group was B (Rh-positive), and he received 250 mL of CP for 2 consecutive days from a donor with ABO blood group A (Rh-positive). The donor's anti-B titer was 1:32. The patient showed clear improvement in respiratory distress and fever symptoms for 3 days after the plasma transfusion. On the third day after plasma transfusion, his PaO2/FiO2 ratio improved to 146, and CXR and fever also improved. There was no evident acute adverse effect of the ABO mismatch. However, 4 days after the plasma transfusion, he presented respiratory distress again. With a sudden oxygen exchange dysfunction, his d-dimer rose to 35.04 μg/mL. As there was no prominent
symptom or laboratory findings that were suggestive of disseminated intravascular coagulation, we initiated intravenous heparin infusion for suspicious pulmonary vein thromboembolism. Colistin inhalation (75 mg every 12 hours) and meropenem (1 gram every 8 hours) were empirically used with extracorporeal membranous oxygenation (ECMO) and dexamethasone (20 mg for 5 days, then 10 mg for 5 days, and tapered in half every 5 days) (Fig. 1). The patient was able to leave after 12 days of ECMO and was discharged without any other complication. When the patient was discharged, all N genes, RdRp, E were not detected in the secretion of the upper respiratory tract. The cut off-value was the 40-CT.
Go to : 
In this case, the improvement after CP was short-lived, and it was difficult to assess the effects of CP clearly when ECMO, steroid, heparinization, and antibiotic were considered to treat re-exacerbation. However, this experience highlighted several points to be addressed for the widespread use of CP.
In Korea, there was a demand for CP therapy during the outbreak of MERS-CoV in 2015 and COVID-19 in 2020. However, the following problems hindered the widespread application of this therapy: 1) a lack of guidelines, 2) a lack of donors, 3) concerns about adverse effects, and 4) difficulties in matching donors and recipients. Guidelines were recently announced on April 13, 2020, for the use of CP for COVID-19 and were distributed to physicians. Although the number of COVID-19 donors seems sufficient compared to the low number of patients with MERS-CoV during the earlier outbreak, concerns about adverse effects and optimal matching of patients remain unaddressed.
ABO blood group mismatching can be a major problem for whole blood transfusion, but not for plasma transfusion. The most important concern among physicians in the use of CP is transfusion-related acute lung injury (TRALI). TRALI is defined as an acute respiratory distress syndrome that occurs within 6 hours of blood administration.13 Some clinicians have vague concerns about TRALI when performing ABO-mismatch CP, but TRALI is rarely associated with ABO mismatch.14 To reduce TRALI, we recommend avoiding previously pregnant female donors.15 Transfusion-associated circulatory overload is another important complication in CP transfusion. In particular, in severe COVID-19 patients, vascular permeability is increased by cytokines16; hence, it is necessary to be careful about volume overload. In our case, the plasma transfusion was performed over 2 days because of concerns regarding volume overload.
Immediate intravascular hemolytic transfusion reactions (IHTR) are considered the most severe complication of ABO-incompatible plasma transfusion. However, the likelihood of IHTR occurring during a transfusion is estimated to be between 1:2,000 and 1:9,000 in patients with severe systemic diseases, and fatalities are extremely rare.17 Although it is advisable to avoid the transfusion of ABO blood group O plasma to individuals with group AB, there is no official contraindication. Quantitative analysis of anti-A and anti-B should be considered in predicting hemolysis. The isoagglutination test is a method for the evaluation of immunoglobulin M levels,18 with a titer above 1:100 indicating a potential for hemolysis.17
The scheduling of the donor is another challenge in the use of plasma therapy for COVID-19. Currently, fresh liquid plasma is collected from the donor at the recipient’s hospital. However, most donors are young and often cannot visit hospitals because of distance or time constraints. Frozen plasma could be considered as a method to overcome these constraints. Frozen plasma also has the advantage of reducing the delay associated with donor preparation and allows for more flexibility in the timing of administration. It may be helpful to administer early in the disease, before patient's neutralizing antibodies to COVID-19 are produced,19 but there is insufficient CP supply to administer to patients with mild symptoms. Therefore, the onset of the acute exacerbation period should be considered as the time point of CP administration. In particular, the immune modulation effect of CP20 may be beneficial early in the exacerbation process. Frozen storage can help with administering CP at the appropriate time.
The distance between the donor candidate and the patient in need of CP may also be a problem. It is not easy to address distance-related challenges as the current Blood Management Law makes it difficult to collect plasma from a donor in a nearby hospital and send it to the hospital where the patient is located. Therefore, the government needs to modify relevant laws to help facilitate the transport of CP between medical institutions. Eventually, the introduction of systems such as CP banks should be considered.
To date, given the ongoing efforts for mass production of antibodies, there is no randomized controlled study of CP, as it is viewed as a temporary and limited treatment. However, it is necessary to keep in mind that new emerging infectious diseases might spread without established treatments and that CP could again be one of the few available options to fight against a new pathogen.
In conclusion, we recommend the implementation of an effective CP system that can combat COVID-19 and other infectious diseases that may emerge in the future.
Go to : 
References
1. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020; 382(8):727–733. PMID: 31978945.

2. Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020; 382(19):1787–1799. PMID: 32187464.
3. Taccone FS, Gorham J, Vincent JL. Hydroxychloroquine in the management of critically ill patients with COVID-19: the need for an evidence base. Lancet Respir Med. 2020; 8(6):539–541. PMID: 32304640.

4. Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020; 395(10236):1569–1578. PMID: 32423584.
5. Yoo JH. Uncertainty about the efficacy of remdesivir on COVID-19. J Korean Med Sci. 2020; 35(23):e221. PMID: 32537956.

6. Gasparyan AY, Misra DP, Yessirkepov M, Zimba O. Perspectives of immune therapy in coronavirus disease 2019. J Korean Med Sci. 2020; 35(18):e176. PMID: 32383371.

7. Ingraham NE, Lotfi-Emran S, Thielen BK, Techar K, Morris RS, Holtan SG, et al. Immunomodulation in COVID-19. Lancet Respir Med. 2020; 8(6):544–546. PMID: 32380023.

8. Khailany RA, Safdar M, Ozaslan M. Genomic characterization of a novel SARS-CoV-2. Gene Rep. 2020; 19:100682.

9. Mair-Jenkins J, Saavedra-Campos M, Baillie JK, Cleary P, Khaw FM, Lim WS, et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. 2015; 211(1):80–90. PMID: 25030060.

10. Casadevall A, Pirofski LA. The convalescent sera option for containing COVID-19. J Clin Invest. 2020; 130(4):1545–1548. PMID: 32167489.

11. Ahn JY, Sohn Y, Lee SH, Cho Y, Hyun JH, Baek YJ, et al. Use of convalescent plasma therapy in two COVID-19 patients with acute respiratory distress syndrome in Korea. J Korean Med Sci. 2020; 35(14):e149. PMID: 32281317.

12. Yoo JH. Convalescent plasma therapy for corona virus disease 2019: a long way to go but worth trying. J Korean Med Sci. 2020; 35(14):e150. PMID: 32281318.

13. Kleinman S, Caulfield T, Chan P, Davenport R, McFarland J, McPhedran S, et al. Toward an understanding of transfusion-related acute lung injury: statement of a consensus panel. Transfusion. 2004; 44(12):1774–1789. PMID: 15584994.

14. Lee JH, Kang ES, Kim DW. Two cases of transfusion-related acute lung injury triggered by HLA and anti-HLA antibody reaction. J Korean Med Sci. 2010; 25(9):1398–1403. PMID: 20808691.

15. Chapman CE, Williamson LM. National blood service TRALI reduction policies: implementation and effect. Transfus Med Hemother. 2008; 35(2):93–96. PMID: 21512634.

16. Moore JB, June CH. Cytokine release syndrome in severe COVID-19. Science. 2020; 368(6490):473–474. PMID: 32303591.

17. Berséus O, Boman K, Nessen SC, Westerberg LA. Risks of hemolysis due to anti-A and anti-B caused by the transfusion of blood or blood components containing ABO-incompatible plasma. Transfusion. 2013; 53(Suppl 1):114S–123S. PMID: 23301963.

18. Ebert RV, Emerson CP Jr. A clinical study of transfusion reactions: the hemolytic effect of group-O blood and pooled plasma containing incompatible isoagglutinins. J Clin Invest. 1946; 25(4):627–638. PMID: 16695355.

19. Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LF. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020; 20(6):363–374. PMID: 32346093.

20. Rojas M, Rodríguez Y, Monsalve DM, Acosta-Ampudia Y, Camacho B, Gallo JE, et al. Convalescent plasma in Covid-19: possible mechanisms of action. Autoimmun Rev. 2020; 19(7):102554. PMID: 32380316.

Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download