INTRODUCTION
The novel coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), has rapidly emerged since December 2019. As of May 24, 2020, a total of 5,204,508 confirmed cases, including 337,687 deaths, have been reported worldwide.
1 Despite the introduction of social distancing to slow the spread in many countries, the outbreak of COVID-19 has overwhelmed healthcare systems in several countries. Inadequate medical resources have warranted the identification of risk factors for the diagnosis and severity of COVID-19. Although early epidemiological studies from China reported the prevalence of comorbidities,
23 and its association with disease severity,
45 the host factors that increase the risk of COVID-19 infection or its severity still need to be identified.
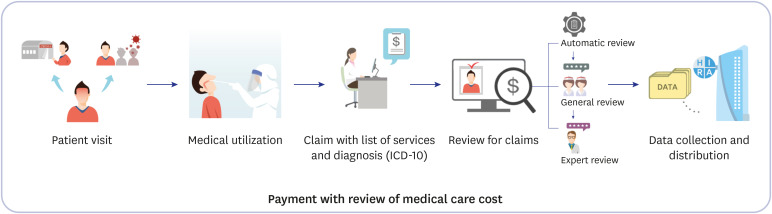
The national health insurance service of Korea registered approximately all COVID-19 patients and test negative controls within the national insurance system. Recently, the Health Insurance Review & Assessment Service (HIRA) of Korea agreed to the share invaluable national health insurance claims data related to COVID-19 for public health purposes. This data is useful for the identification of underlying comorbidities which are associated with the diagnosis and severity of COVID-19.
We conducted a retrospective case-control study, examining the effect of various underlying comorbidities on the risk of infection and severity of COVID-19 using data from the nationwide medical insurance claim database in Korea.
DISCUSSION
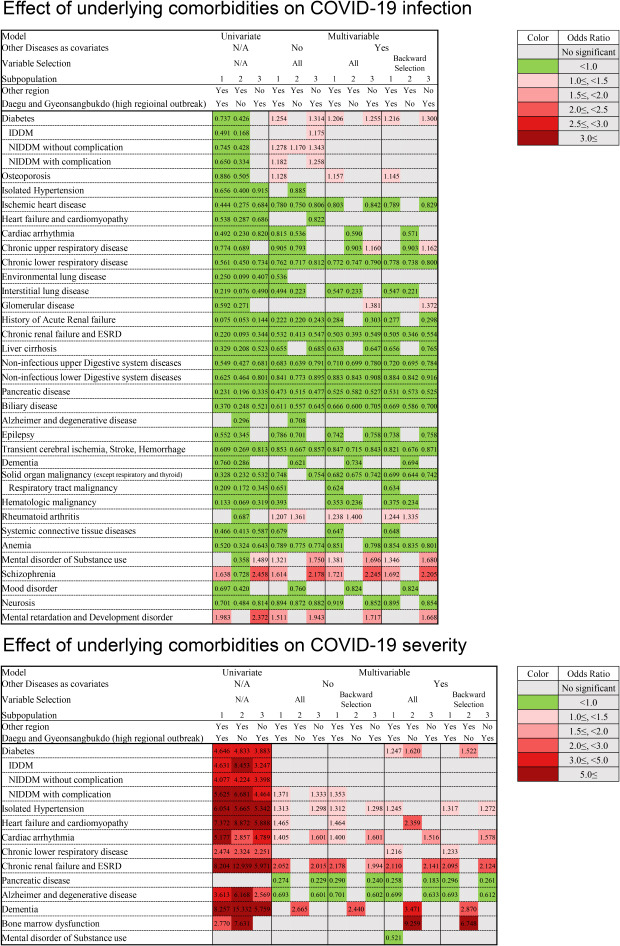
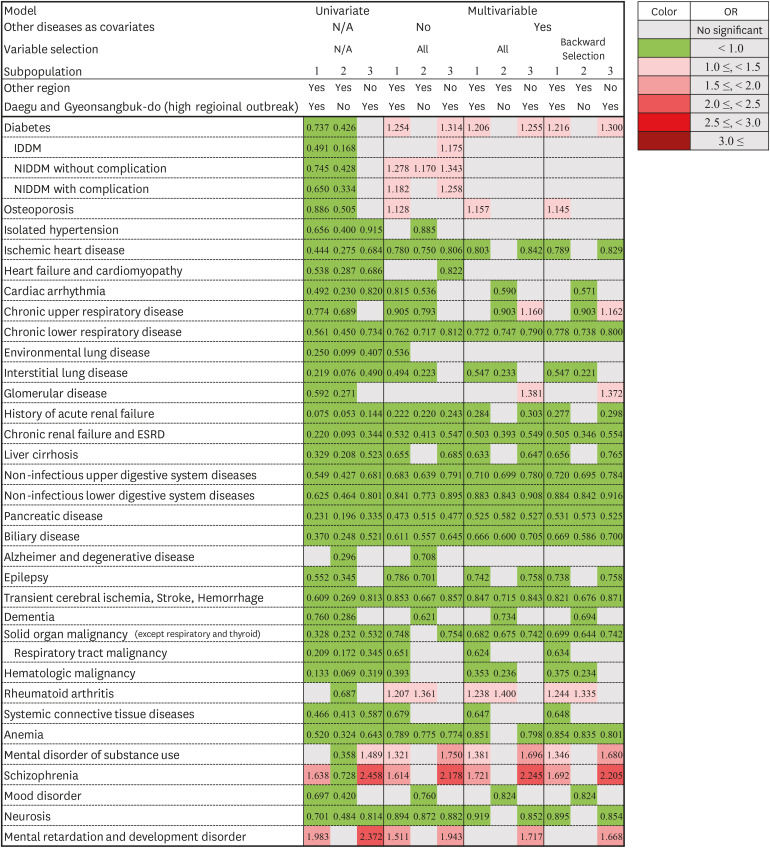
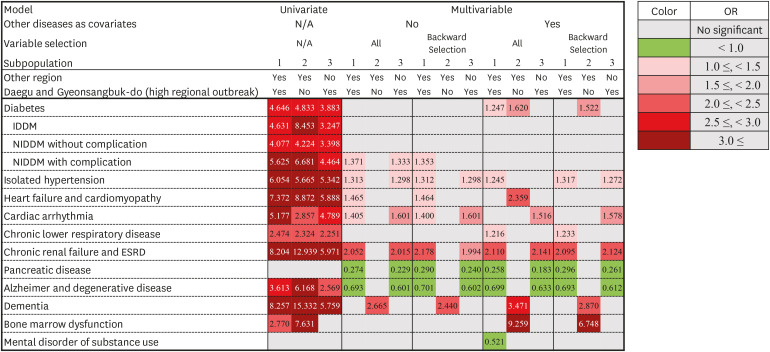
In this study, we identified the possible comorbidities that might be associated with the risk of COVID-19 infection and its severity. Diabetes, osteoporosis, rheumatoid arthritis, substance use, and schizophrenia showed significant associations with the diagnosis of COVID-19. The patterns of comorbidities associated with the occurrence of COVID-19 might be different between high and non-high regional outbreak areas. Diabetes, chronic upper respiratory diseases, glomerular disease, substance use, schizophrenia, mental retardation, and developmental disorders were associated with community outbreak (DG area), whereas rheumatoid arthritis was associated with containment area (e.g., institutional outbreak). Diabetes, isolated hypertension, chronic lower respiratory disease, CRF, and ESRD were associated with severe COVID-19. Moreover, diabetes was strongly associated with the diagnosis and severity of COVID-19.
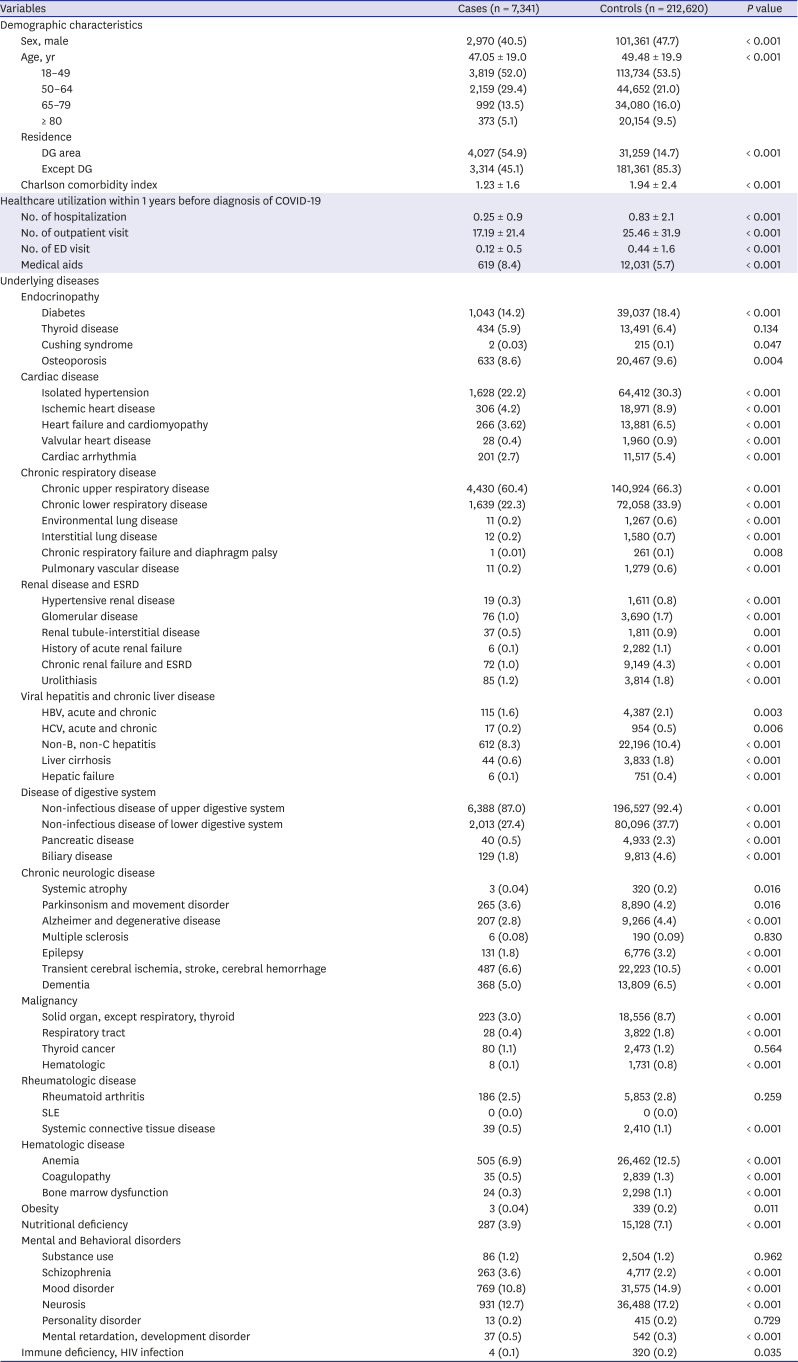
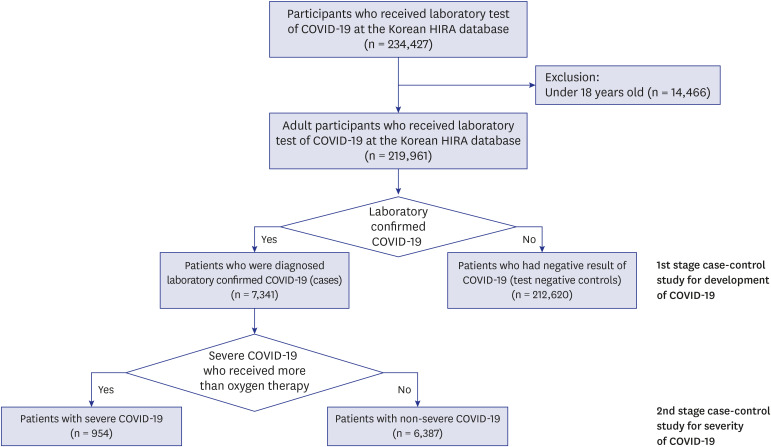
When interpreting the results of this study, it is necessary to consider the demographic differences between the case and control groups. Most diseases, except schizophrenia, mental retardation, and developmental disorders, showed a higher prevalence in the control group than in the case group (
Table 1). This suggested that more tests for COVID-19 were performed in people at a risk of febrile respiratory illness, regardless of the COVID-19 pandemic.
91011 Therefore, we presumed that the negative association in univariate analysis related to COVID-19 occurrence was based on biased baseline demographics and found that this selection bias was alleviated in the multivariate analysis (
Fig. 3). Moreover, we compared the laboratory confirmed cases to the RT-PCR-negative control groups and conducted subgroup analysis according to high regional outbreak areas where this bias would have been less affected. As a result, the comorbidities that increased the risk of diagnosis of COVID-19 were more meaningful. Therefore, we found that diabetes, osteoporosis, and rheumatoid arthritis might be risk factors for the diagnosis of COVID-19.
In the case of psychological disorders including substance use, schizophrenia, mental retardation, and developmental disorders, consideration as risk factors for COVID-19 was unjustifiable because this might be correlated with the large clustered outbreak in hospitalized patients who were admitted in closed wards for psychological treatment. Even considering these epidemiological characteristics, some early case reports on the COVID-19 outbreak suggested that differences in socioeconomic levels may influence SARS-CoV-2 infection.
12 It might be suggested that the accompanying low socioeconomic status might increase the risk of developing COVID-19 rather than schizophrenia or a psychological disorder itself.
13 This study also found that rheumatoid was associated with the diagnosis of COVID-19. Although the impact of immunosuppression on the diagnosis of COVID-19 remains unclear, it is suggested that the long-term use of steroids or immunosuppressants may increase susceptibility to COVID-19.
In spite of a potential selection bias, the result suggested the possibility of chronic illness including ischemic heart disease, cardiac arrhythmia, chronic upper and lower respiratory disease, chronic renal disease, liver cirrhosis, non-infectious upper and lower digestive disease, pancreatic disease, biliary disease, epilepsy, cerebrovascular disease, dementia, malignancy, systemic connective tissue, anemia, and neurosis being associated with a decreased risk of COVID-19. Patients with these conditions might have reduced social activity, which may reduce the possible risk of exposure to SARS-CoV-2. However, this is not clear, and more precise research is needed.
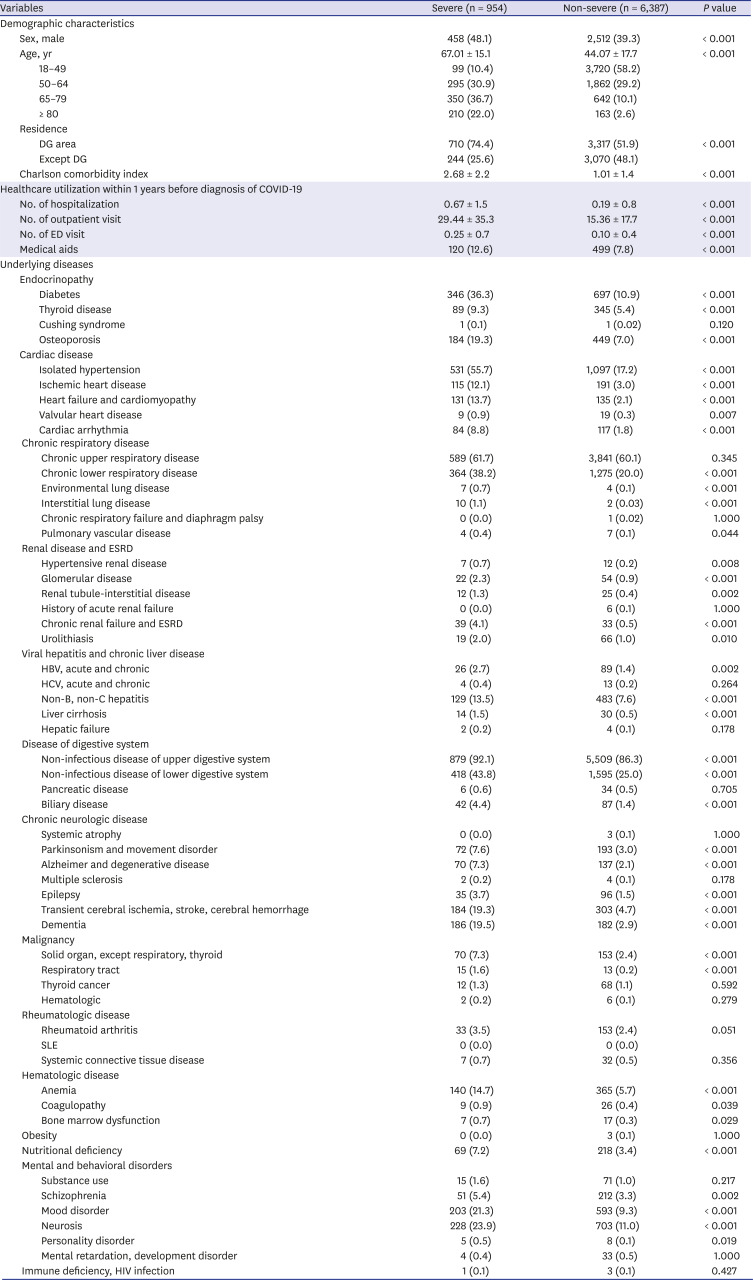
In early epidemiological studies of COVID-19, it was reported that comorbidities including diabetes, hypertension, and chronic respiratory disease, except for malignancy, were related to disease severity or death
45141516 as observed in this study. We also found that CRF and ESRD were significant risk factors for severe COVID-19. In addition, heart failure, cardiomyopathy, and cardiac arrhythmia might be associated with the severity of COVID-19. Human angiotensin-converting enzyme 2 (ACE2) is a functional receptor of SARS-CoV-2,
17 and the heart is a tissue rich in ACE receptors along with the lungs. Cases of COVID-19 related to myocarditis were reported in China
18 and Korea,
19 and myocarditis was also reported as a risk factor for severe COVID-19.
3
In this study, pancreatic disease, Alzheimer's disease, and degenerative diseases were associated with a decreased risk of severe COVID-19. Although the detailed mechanism of this protective effect remains unclear, it is suggested that the possible protective effect may be attributable to drugs used to treat these diseases. For example, camostat mesylate which is widely used protease inhibitor in chronic pancreatic disease might have protective role in COVID-19. TMPRSS2 is a serine protease that primes the spike protein of human coronaviruses and facilitates its entry into the host cell.
20 Camostat mesylate was effective in SARS-CoV infected mouse model,
21 and it is suggested as a potential therapeutic option in COVID-19.
2223
This study has a few limitations. First, this study was limited to data from the nationwide claims database of subjects who underwent laboratory testing for COVID-19. Thus, the data of patients who were tested via “Drive Through” or local health centers and were treated at non-medical facilities was not included in this study. Therefore, the actual population affected by the disease was different from that of the analyzed population, which was used in this study. Despite this limitation, this study was conducted only for those who underwent the laboratory test for COVID-19 as per the insurance claims database. Therefore, the negative control group had a confirmed a negative result of SARS-CoV-2 infection. Thus, the comparison between this negative control group and case group helped in proper evaluation of the risk factors for COVID-19 occurrence. Another limitation was the inability of the data source to provide information on the severity of the comorbidities. Finally, we could not evaluate the detailed mechanism underlying the relationship between comorbidities and the diagnosis or severity of COVID-19. However, most comorbidities identified in each individual's health insurance claims data could be used for this study. It may be possible to discover previously unknown or unexpected risk factors based on a data driven approach.
In this retrospective case control study, we suggest that diabetes, osteoporosis, rheumatoid arthritis, disorder owing to substance use, and schizophrenia might be risk factors for COVID-19. Besides, hypertension, chronic lower respiratory disease, CRF, and ESRD are associated with severe COVID-19. In addition, diabetes is associated with the occurrence and severity of COVID-19.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download