INTRODUCTION
In early December 2019, cases of pneumonia caused by a new type of coronavirus infection appeared in the city of Wuhan, Hubei Province, China.
1 The disease quickly spread to the other parts of China and many other countries on the six continents. The World Health Organization (WHO) named the disease caused by the new coronavirus as coronavirus disease-2019 (COVID-19) and announced that COVID-19 had reached the pandemic status.
23 According to data from WHO, there were 4,619,477 confirmed cases of COVID-19 and 311,847 confirmed deaths due to COVID-19 as of May 18, 2020 worldwide.
4
Since the first case was reported on 11 March in Turkey (much later than in many European countries), the number of cases has increased very quickly and reached the peak in mid-April.
45 Unsurprisingly, most of these cases occurred in Istanbul (1 hour away from Bursa), the country's most populous city, with a population of over 16 million. But there is no detailed information about gender, age, clinical stage and prognosis of identified cases.
67
The first data about the disease mainly focused on severe respiratory symptoms observed in adults, and this situation led to a lack of data to explain the different course of COVID-19 in children.
8 The clinical spectrum of COVID-19 ranges from asymptomatic to critical patients. Fever and cough are the most common symptoms in symptomatic adults.
9 In pediatric patients, clinical findings are not typical and relatively milder than those in adult patients. While these patients are mostly asymptomatic or have upper respiratory symptoms including fever, dry cough, weakness, runny nose, and nasal congestion, some patients may present with gastrointestinal symptoms such as abdominal pain, nausea-vomiting, and diarrhea.
10 Their clinical findings and prognosis are generally good. It was reported that most cases recovered completely within 1 to 2 weeks. It was reported that acute respiratory distress syndrome, septic shock, metabolic acidosis, and coagulation disorders might occur in rare cases.
11
While there are several studies involving adult patients diagnosed with COVID-19, a limited number of studies addressed COVID-19 in the pediatric age group. In this study, we aimed to collect the current methods of protection from and treatment for this unfamiliar pathogen and to contribute to the urgently needed evidence-based knowledgebase for COVID-19 in children by examining the epidemiological, laboratory, radiological, and therapeutic features of children diagnosed with the disease after admission to our hospital retrospectively.
METHODS
Study design and data collection
The study was conducted as a retrospective observational cohort study at a single center (the Department of Pediatrics at Bursa City Hospital which is located in a large city in northwest Turkey). The patients under the age of 18 years, who had confirmed diagnosis of COVID-19 between March 5 and May 5, 2020, and had been followed up in the outpatient or inpatient clinics, were included. The patients’ medical records in the hospital’s electronic database were retrospectively analyzed. The patients over the age of 18 years, whose files could not be reached or who had serious data deficits, were not included.
All patients were evaluated in terms of their epidemiological and demographic characteristics, laboratory and radio-diagnostic tests, and treatment features and outcomes. The data were reviewed by two trained physicians.
Procedures
The diagnosis, monitoring, and treatment for COVID-19 were based on the guidelines published by the Turkish Ministry of Health's Scientific Committee on COVID-19 (
Table 1). For the reverse-transcription polymerase chain reaction (RT-PCR) test for severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), combined naso-oropharyngeal swabs were taken from the patients who met the criteria laid out in the guidelines. The samples were tested in Ankara Public Health Laboratory of the Ministry of Health until April 13, 2020, and later in our hospital laboratory. Viral analyses and real-time (Q) PCR experiments were applied to sample of the patient. The RNA was isolated using vNAT solution (Bioeksen, Istanbul, Turkey). For all reactions, Rotor-Gene Q (Qiagen, Antwerp, Belgium) instrument and Biospeedy SARS-CoV-2 RT-qPCR kit (Bioeksen) were used. The kit is performed in one step with targeting the RdRp (RNA-dependent RNA polymerase) gene fragment reverse transcription (RT) and rt PCR (QPCR) (RT-QPCR). The data was analyzed using Rotor-Gene Q Software.
Table 1
Sampling criteria for suspected COVID-19 cases

|
Criteria |
Description |
|
Epidemiological features (I) |
Having a household member hospitalized with the diagnosis of respiratory tract infection within the last 14 days |
|
Having a household member diagnosed with COVID-19 |
|
Having a household member with fever and cough or suffering from respiratory distress with or without fever |
|
Having a contact history with someone diagnosed with COVID-19 |
|
Complaints and findings (II) |
Presence of a fever or a measured temperature of 38.0°C or above |
|
Presence of lung percussion and auscultation findings |
|
Presence of tachypnea |
|
Presence of new onset cough |
|
Having an oxygen saturation of 92% or lower in room air |
|
COVID-19 RT-PCR test is requested in the following cases |
The presence of at least one of each of I and II |
|
The presence of at least two of II (for each item, the relation to another item cannot be shown) |
|
Presence of two or more people with COVID-19 diagnosis in the same household |
|
Infants under 9 months who have a mother diagnosed with COVID-19 |
At the time of application, complete blood count, serum biochemical test (including renal and liver functions, lactate dehydrogenase [LDH], and electrolytes), myocardial enzymes, coagulation profile, erythrocyte sedimentation rate, C-reactive protein (CRP), and procalcitonin were studied for these patients. Nasopharyngeal swabs were taken for the influenza test. Blood culture was taken at the fever period from the patients with suspected secondary bacterial infection. Their chest X-rays were also taken. Chest computed tomography (CT) was performed in the patients whose respiratory findings could not be explained by the chest X-ray or in those with poor clinical prognosis.
SARS-CoV-2 positive patients were hospitalized and followed up in inpatient clinics during March when the COVID-19 outbreak started in Turkey. With the recommendations of the Scientific Committee for COVID-19, some patients were allowed to be isolated at home starting in April. Patients with any of the following conditions were hospitalized: tachypnea (≥ 70 breaths per minutes for infants aged < 1 year; ≥ 50 breaths per minutes for children aged > 1 year), hypoxia (oxygen saturation < 92%), lack of consciousness, depression, coma, convulsions, dehydration, difficulty feeding, gastrointestinal dysfunction, myocardial injury, elevated liver enzymes, coagulation dysfunction, rhabdomyolysis, and any other manifestations suggesting injuries to vital organs. From the patients who were followed up at the hospital, RT-PCR swab samples were taken at the 48th hour of hospitalization, which was continued until a negative test result. The discharge criteria were the absence of fever for at least 48 hours, a substantial improvement in laboratory tests, the clinical remission of respiratory symptoms, and at least one negative RT-PCR test result for SARS-CoV-2. The outpatients were asked for their complaints via regular phone calls, and they were not retested for SARS-CoV-2 unless additional complaints or findings developed.
The treatment protocol for the inpatients included general supportive therapy, the monitoring of lung, liver, kidney, and heart functions, an active control of high fever, antibiotherapy, antiviral therapy if necessary (the administration of oseltamivir in the patients who were positive for influenza) and, supplemental oxygen therapy if necessary (oxygen through a nasal cannula at a rate of 2–4 L/min for the patients with saturation < 92% in the absence of oxygen support), systemic corticosteroids, and inhaled corticosteroids. Ceftriaxone/Cefotaxime was given to the patients with suspected secondary bacterial infection; all others were given azithromycin. In outpatients, amoxycillin clavulanate and azithromycin treatments were given for secondary bacterial infection susceptibility.
Statistical analysis
The data were presented as frequency (number) and percentage (%) for categorical variables and as mean ± standard deviation or median (interquartile range) for continuous variables, as appropriate. χ2 test or Fisher's exact tests were used, as appropriate, to compare the qualitative variables. Two-group comparisons were performed using the Mann Whitney U test for other data. All tests were two-tailed; P values of < 0.05 were considered to indicate statistical significance, applying Bonferroni correction when appropriate. Statistical analyses were performed by using SPSS version 21.0 (SPSS Inc., Chicago, IL, USA).
Ethics statement
The present study protocol was reviewed and approved by the Institutional Review Board (IRB) at Uludağ University (IRB No. 2011-KAEK-26/282). Informed consent was waived.
RESULTS
At the time interval covered by the study, 6,258 visits were made to the pediatric emergency department of our hospital. Naso-oropharyngeal swab samples for RT-PCR were taken from 367 patients (5.8%) suspected of having COVID-19; 79 (21.5%) patients were positive for SARS-CoV-2. The swab samples were taken from 22 suspected newborns in the neonatal intensive care unit; four were born to SARS-CoV-2-positive mothers, and 16 were born to mothers suspected of having the virus. Two newborns were tested due to having contact history with a SARS-CoV-2-positive parents at home; only these two were positive (9%). Of those who were positive, 44 patients (54%) were hospitalized and followed up in the inpatient clinic.
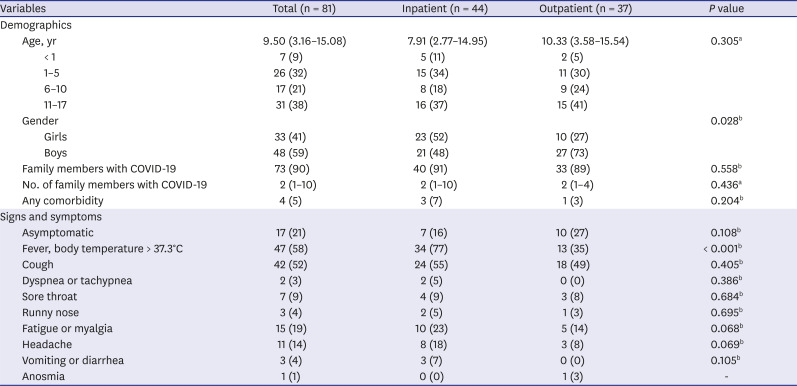
The median age was 9.50 years (3.16–15.08 years) in the study group (
Table 2). The boys/girls ratio was 1.45 (48/33). At least one SARS-CoV-2-positive family member was present in 90% of the cases. Four patients had comorbid conditions: two were being followed up for cerebral palsy, one for type-1 diabetes mellitus, and one for asthma.
Table 2
Demographic and clinical features of pediatric patients with COVID-19
|
Variables |
Total (n = 81) |
Inpatient (n = 44) |
Outpatient (n = 37) |
P value |
|
Demographics |
|
|
|
|
|
Age, yr |
9.50 (3.16–15.08) |
7.91 (2.77–14.95) |
10.33 (3.58–15.54) |
0.305a
|
|
|
< 1 |
7 (9) |
5 (11) |
2 (5) |
|
|
1–5 |
26 (32) |
15 (34) |
11 (30) |
|
|
6–10 |
17 (21) |
8 (18) |
9 (24) |
|
|
11–17 |
31 (38) |
16 (37) |
15 (41) |
|
Gender |
|
|
|
0.028b
|
|
|
Girls |
33 (41) |
23 (52) |
10 (27) |
|
|
Boys |
48 (59) |
21 (48) |
27 (73) |
|
Family members with COVID-19 |
73 (90) |
40 (91) |
33 (89) |
0.558b
|
|
No. of family members with COVID-19 |
2 (1–10) |
2 (1–10) |
2 (1–4) |
0.436a
|
|
Any comorbidity |
4 (5) |
3 (7) |
1 (3) |
0.204b
|
|
Signs and symptoms |
|
|
|
|
|
Asymptomatic |
17 (21) |
7 (16) |
10 (27) |
0.108b
|
|
Fever, body temperature > 37.3°C |
47 (58) |
34 (77) |
13 (35) |
< 0.001b
|
|
Cough |
42 (52) |
24 (55) |
18 (49) |
0.405b
|
|
Dyspnea or tachypnea |
2 (3) |
2 (5) |
0 (0) |
0.386b
|
|
Sore throat |
7 (9) |
4 (9) |
3 (8) |
0.684b
|
|
Runny nose |
3 (4) |
2 (5) |
1 (3) |
0.695b
|
|
Fatigue or myalgia |
15 (19) |
10 (23) |
5 (14) |
0.068b
|
|
Headache |
11 (14) |
8 (18) |
3 (8) |
0.069b
|
|
Vomiting or diarrhea |
3 (4) |
3 (7) |
0 (0) |
0.105b
|
|
Anosmia |
1 (1) |
0 (0) |
1 (3) |
- |
The most frequent symptoms at the time of admission were fever (58%), cough (52%), and fatigue or myalgia (19%). No neurological findings or signs of cardiac, liver, or renal failure were found in the patients.
A comparison of the inpatients and outpatients for the demographics and the complaints at the time of admission indicated that women gender (P = 0.02) and fever complaints (P < 0.001) were significantly more frequent in hospitalized patients.
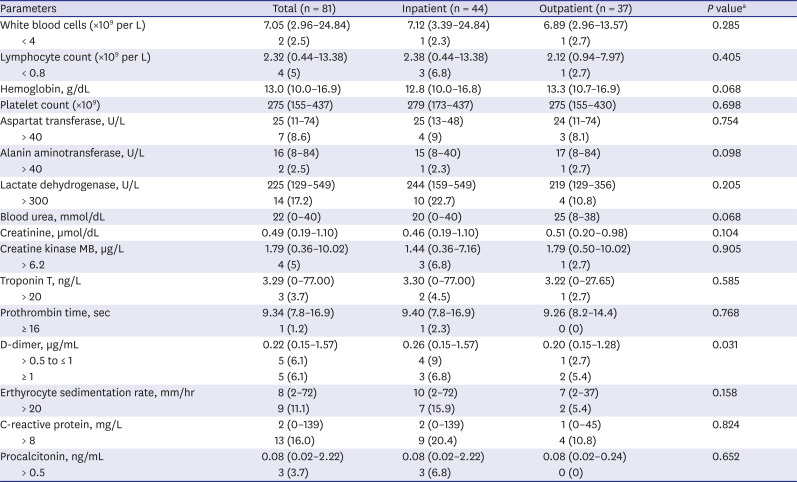
The laboratory results of the patients were shown in
Table 3. Abnormal results were decreased lymphocytes (2.5%, n = 2), leucopenia (5%, n = 4), and increased LDH (17.2%, n = 14), CRP (16%, n = 13), procalcitonin (3.7%, n = 3), and D-dimer (12.3%, n = 10). The inpatients had significantly higher levels of D-dimer than the outpatients (
P = 0.03).
Table 3
Laboratory findings of pediatric patients with coronavirus disease 2019
|
Parameters |
Total (n = 81) |
Inpatient (n = 44) |
Outpatient (n = 37) |
P valuea
|
|
White blood cells (×109 per L) |
7.05 (2.96–24.84) |
7.12 (3.39–24.84) |
6.89 (2.96–13.57) |
0.285 |
|
< 4 |
2 (2.5) |
1 (2.3) |
1 (2.7) |
|
Lymphocyte count (×109 per L) |
2.32 (0.44–13.38) |
2.38 (0.44–13.38) |
2.12 (0.94–7.97) |
0.405 |
|
< 0.8 |
4 (5) |
3 (6.8) |
1 (2.7) |
|
Hemoglobin, g/dL |
13.0 (10.0–16.9) |
12.8 (10.0–16.8) |
13.3 (10.7–16.9) |
0.068 |
|
Platelet count (×109) |
275 (155–437) |
279 (173–437) |
275 (155–430) |
0.698 |
|
Aspartat transferase, U/L |
25 (11–74) |
25 (13–48) |
24 (11–74) |
0.754 |
|
> 40 |
7 (8.6) |
4 (9) |
3 (8.1) |
|
Alanin aminotransferase, U/L |
16 (8–84) |
15 (8–40) |
17 (8–84) |
0.098 |
|
> 40 |
2 (2.5) |
1 (2.3) |
1 (2.7) |
|
Lactate dehydrogenase, U/L |
225 (129–549) |
244 (159–549) |
219 (129–356) |
0.205 |
|
> 300 |
14 (17.2) |
10 (22.7) |
4 (10.8) |
|
Blood urea, mmol/dL |
22 (0–40) |
20 (0–40) |
25 (8–38) |
0.068 |
|
Creatinine, µmol/dL |
0.49 (0.19–1.10) |
0.46 (0.19–1.10) |
0.51 (0.20–0.98) |
0.104 |
|
Creatine kinase MB, µg/L |
1.79 (0.36–10.02) |
1.44 (0.36–7.16) |
1.79 (0.50–10.02) |
0.905 |
|
> 6.2 |
4 (5) |
3 (6.8) |
1 (2.7) |
|
Troponin T, ng/L |
3.29 (0–77.00) |
3.30 (0–77.00) |
3.22 (0–27.65) |
0.585 |
|
> 20 |
3 (3.7) |
2 (4.5) |
1 (2.7) |
|
Prothrombin time, sec |
9.34 (7.8–16.9) |
9.40 (7.8–16.9) |
9.26 (8.2–14.4) |
0.768 |
|
≥ 16 |
1 (1.2) |
1 (2.3) |
0 (0) |
|
D-dimer, µg/mL |
0.22 (0.15–1.57) |
0.26 (0.15–1.57) |
0.20 (0.15–1.28) |
0.031 |
|
> 0.5 to ≤ 1 |
5 (6.1) |
4 (9) |
1 (2.7) |
|
≥ 1 |
5 (6.1) |
3 (6.8) |
2 (5.4) |
|
Erthyrocyte sedimentation rate, mm/hr |
8 (2–72) |
10 (2–72) |
7 (2–37) |
0.158 |
|
> 20 |
9 (11.1) |
7 (15.9) |
2 (5.4) |
|
C-reactive protein, mg/L |
2 (0–139) |
2 (0–139) |
1 (0–45) |
0.824 |
|
> 8 |
13 (16.0) |
9 (20.4) |
4 (10.8) |
|
Procalcitonin, ng/mL |
0.08 (0.02–2.22) |
0.08 (0.02–2.22) |
0.08 (0.02–0.24) |
0.652 |
|
> 0.5 |
3 (3.7) |
3 (6.8) |
0 (0) |
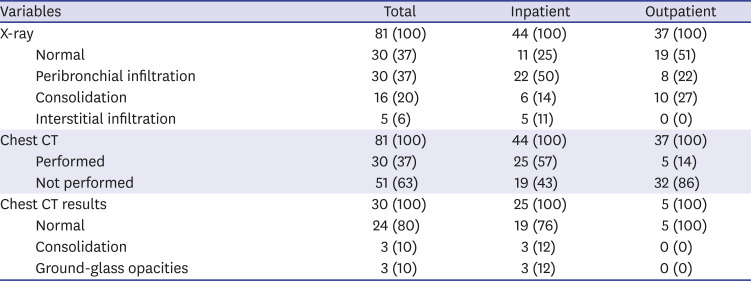
In radiology, chest X-rays were normal in 30 patients (37%) while 51 patients (63%) had potentially pathological findings (
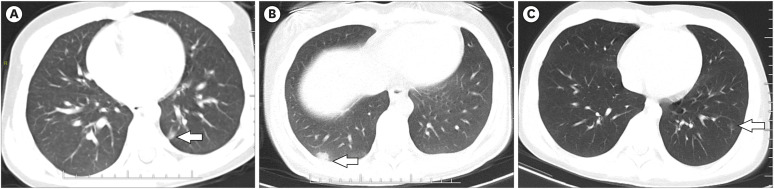
Table 4). 30 (59%) who underwent chest CT was from 51 patients with abnormal findings, and 3 (10%) patients with consolidation and 3 (10%) with ground-glass opacities (
Fig. 1).
Table 4
Radiological features of pediatric patients with coronavirus disease 2019
|
Variables |
Total |
Inpatient |
Outpatient |
|
X-ray |
81 (100) |
44 (100) |
37 (100) |
|
Normal |
30 (37) |
11 (25) |
19 (51) |
|
Peribronchial infiltration |
30 (37) |
22 (50) |
8 (22) |
|
Consolidation |
16 (20) |
6 (14) |
10 (27) |
|
Interstitial infiltration |
5 (6) |
5 (11) |
0 (0) |
|
Chest CT |
81 (100) |
44 (100) |
37 (100) |
|
Performed |
30 (37) |
25 (57) |
5 (14) |
|
Not performed |
51 (63) |
19 (43) |
32 (86) |
|
Chest CT results |
30 (100) |
25 (100) |
5 (100) |
|
Normal |
24 (80) |
19 (76) |
5 (100) |
|
Consolidation |
3 (10) |
3 (12) |
0 (0) |
|
Ground-glass opacities |
3 (10) |
3 (12) |
0 (0) |
Fig. 1
Chest computed tomography images of children with coronavirus disease 2019. (A) 5.5-year-old boy showing 8 × 5 mm ground-glass opacity in lower lobe of the left lung (arrow). (B) 17-year-old girl showing 13 × 10 mm ground-glass opacity in lower lobe of the right lung (arrow). (C) 12-year-old boy showing multiple ground-glass opacities in lower lobe of the left lung (arrow).
There were two newborn patients (3%) with positive test results during the study period; one was 11 days old, the other was 8 days old. They were asymptomatic and had no pathological findings in laboratory tests and chest X-rays. They were discharged without any additional treatment after nine and eight days of monitoring, respectively.
Antibiotics were used in 95% of the patients who were hospitalized (
Table 5). None of the patients received oseltamivir treatment since none had a positive influenza test. One patient (2%) received oxygen through a nasal cannula, one patient (2%) received inhaled corticosteroids, and two patients (5%) with bronchiolitis did not respond adequately to inhalation therapy received low dose systemic corticosteroid treatment (prednisolone 1 mg/kg per day for three days) in a negative pressure room.
Table 5
Treatment and outcome features of pediatric inpatients with coronavirus disease 2019
|
Variables |
Total (n = 44) |
≤ 5 yr (n = 20) |
> 5 yr (n = 24) |
P value |
|
Treatments |
|
|
|
|
|
Antibiotics |
42 (95) |
18 (90) |
24 (100) |
0.204a
|
|
|
Ceftriaxone/cefotaxime |
8 (18) |
6 (30) |
2 (8) |
0.078a
|
|
|
Azitromycine |
42 (95) |
18 (90) |
24 (100) |
0.205a
|
|
Antiviral treatment |
0 (0) |
- |
- |
- |
|
Corticosteroids |
2 (5) |
2 (10) |
0 (0) |
- |
|
Intravenous immunoglobin |
0 (0) |
- |
- |
- |
|
Nasal cannula oxygen therapy |
1 (2) |
1 (5) |
0 (0) |
- |
|
Inhaled corticosteroids |
1 (2) |
1 (5) |
0 (0) |
- |
|
Diagnosis |
|
|
|
|
|
Asymptomatic infection |
7 (16) |
5 (25) |
2 (8) |
0.154a
|
|
Upper respiratory infection |
4 (9) |
3 (15) |
1 (4) |
0.085a
|
|
Pneumonia |
33 (75) |
12 (60) |
21 (88) |
0.068a
|
|
Outcomes |
|
|
|
|
|
Intensive care |
2 (5) |
2 (10) |
0 (0) |
- |
|
Time taken to become SARS-CoV-2 RT-PCR-negative, days |
5 (3–10) |
6 (3–10) |
4 (3–9) |
0.037b
|
|
No. of samples taken to get negative SARS-CoV-2 RT-PCR results |
3 (2–4) |
3 (2–4) |
2 (2–4) |
0.061b
|
|
Total duration of fever, days |
1 (0–3) |
1 (0–3) |
1 (0–3) |
0.404b
|
|
Total duration of cough, days |
1 (0–2) |
1 (0–2) |
1 (0–2) |
0.358b
|
|
Total duration of dyspnea or tachypnea, days |
1 (0–2) |
NA |
0.5 (0–2) |
- |
|
Total duration of sore throat, days |
1 (0–2) |
NA |
1 (0–2) |
- |
|
Total duration of runny nose, days |
1 (0–1) |
NA |
1 (0–1) |
- |
|
Total duration of fatigue or myalgia, days |
1 (0–1) |
NA |
1 (0–1) |
- |
|
Total duration of headache, days |
1 (0–2) |
NA |
0.5 (0–2) |
- |
|
Total duration of vomiting or diarrhea, days |
1 (0–2) |
1 (0–2) |
1 (0–2) |
0.359b
|
|
Total duration of hospitalization, days |
5 (4–10) |
6 (4–10) |
5 (4–7) |
0.018b
|
|
Total duration in intensive care unit, days |
8.5 (8–9) |
8.5 (8–9) |
NA |
- |
Seven patients (16%) were asymptomatic at the time of admission. Four (9%) had the symptoms of upper respiratory tract infection, and 33 (75%) had pneumonia findings. None of the patients needed intensive care except for the newborns. The median time to turn SARS-CoV-2 negative in the RT-PCR test was 5 (3–10) days. The median value for the number of samples taken to get negative SARS-CoV-2 RT-PCR results was 3 (2–4). The median length of hospital stay was 5 (4–10) days. The time to turn SARS-CoV-2 negative in RT-PCR test and the length of hospital stay were significantly longer for those aged five years or younger compared to the others (P = 0.037, P = 0.01).
DISCUSSION
In this study, we have reported the data for 81 children diagnosed with COVID-19 at a single center since the beginning of the outbreak in Turkey, the country with the eighth highest number of confirmed COVID-19 cases in the world, in order to contribute to the limited knowledge base about pediatric COVID-19 cases. All of the children in the study, including those with comorbid conditions, had recovered; they had no sequelae until the submission of this report.
SARS-CoV-2 is a highly contagious pathogen that commonly causes pneumonia in infected patients.
10 In Turkey, the COVID-19 outbreak started later than in most other countries affected by the pandemic. However, the outbreak exhibited a rapid growth, reaching a total of 148,000 cases on May 18 (in 68 days). Although the number of cases was high, the number of recorded deaths stayed relatively low compared to most other countries where the SARS-CoV-2 infection has been widespread (4,096 deaths, 2.7% death rate).
4
Compared to adults, COVID-19 is more distinctive and milder in children in terms of its clinical symptoms and outcomes.
12 The mechanisms that could explain this situation have not been fully elucidated, and some speculations have been made on the subject.
10 One speculation was that, unlike adults, children are less likely to have an excessive immune response and cytokine storm.
11 The angiotensin-converting enzyme 2 (ACE2) proteins, which are highly expressed in alveolar type 2 cells, are fewer and immature in children compared to those in adults.
13 Besides, children are often exposed to other viral or bacterial infections in daycare and school settings. It has been assumed that a previous exposure to milder respiratory pathogens could train the host immune system to defend against coronavirus.
14 In accordance with the literature, none of our patients needed intensive care except for two patients in the neonatal group, and all of our patients were discharged in good conditions.
Fever was the most common symptom in our patients. While cough and pharyngitis were more frequent in some studies, fever was more common in other studies.
121516 Approximately 10% of the infected children may initially show gastrointestinal symptoms such as diarrhea, abdominal pain, and vomiting, making it difficult to distinguish COVID-19 from other common childhood diseases.
1516 In our study, gastrointestinal symptoms were less frequent (4%). No neurological finding or sign of cardiac, liver, or renal failure was observed in our patients.
The laboratory findings of the pediatric patients with COVID-19 are non-specific. Lymphopenia is one of the most common hematological abnormalities.
10 In adult patients, a marked or progressive decrease in peripheral blood absolute lymphocyte count (ALC) was observed in the acute phase of COVID-19.
17 However, the ALC is within the normal range in most pediatric COVID-19 patients.
1516 Other abnormal laboratory results include increased liver, muscle, and cardiac enzymes, LDH, CRP, procalcitonin, and D-dimer.
1618
Similar to the pulmonary involvement due to previous coronavirus infections, typical radiological findings in COVID-19 patients were consolidation, which indicated parenchymal destruction, and ground-glass appearance. While these involvements have mostly been observed in the adult population with low immunity, they are also found in pediatric patients. These findings have typically peripheral, bilateral, and multifocal distribution, prominently in the lower lobe.
19 In our study, pulmonary consolidation was found in three patients (10%), and ground-glass opacity was found in three patients (10%) among those 30 patients who had chest CT. These findings support the previous data that pulmonary involvement was mild in children with COVID-19.
Young children are more represented in the pediatric population infected with SARS-CoV-2. Of the patients treated in Wuhan Children's Hospital, 18% were infants under one-year-old. In another large-scale study, the frequency of serious and critical disease was 10.6% in those under the age of 1, 7.3% in those between 1-5 years old, 4.2% in those between 6-10 years old, and 7.1% in those between 11-17 years old.
1620 In our study, the need for intensive care occurred only in two patients who were newborns. Although the patients did not experience any complications, the time to reach negative RT-PCR results for SARS-CoV-2 and the length of hospital stay were found to be longer in those under the age of 5 compared to older patients.
Many pregnant women were also infected in the outbreak in Wuhan, but only a small portion (9%) of babies born to these mothers were positive for SARS-CoV-2 through vertical transmission. No prenatal transmission was observed in the three newborns born to infected mothers in our study. Perinatal infection can contribute to adverse events in newborns such as fetal distress, preterm labor, and newborn respiratory distress.
21 Both newborns who were followed throughout the study period were asymptomatic, and no pathological feature was found in laboratory tests or chest radiographs. They were discharged after monitoring without any additional treatment.
The publications that describe the management of pediatric patients with COVID-19 have mostly referred to supportive therapy, including oxygen therapy and the antibiotics for bacterial superinfections.
1018 However, some studies have proposed various antiviral treatments when needed. Among these, antiviral drugs such as ribavirin, lopinavir, and ritonavir were recommended based on the experience in severe acute respiratory syndrome (SARS) and the Middle East respiratory syndrome.
22 Some studies are suggesting that hydroxychloroquine and azithromycin treatments reduced the SARS-CoV-2 viral load.
1023 Animal studies and early clinical experience with the use of remdesivir in adults are promising, and extensive randomized controlled studies are underway to assess the effectiveness of this treatment.
24 The role of corticosteroids has not been proven yet; while current international consensus and WHO are against the use of corticosteroids, the Chinese guidelines recommend a short-term treatment with low to medium dose corticosteroids in COVID-19.
2526 Other therapeutics recommended for treatment are umifenovir (an antiviral drug available in Russia and China) and intravenous immunoglobulins, interferons, and the administration of plasma isolated from the patients who had previously recovered from COVID-19.
2728 However, there is still a need for more evidence to recommend these therapies.
There are some limitations to the current study, which presented the observational data from a single-center with a limited sample size. Therefore, the limited sample size might have prevented drawing definitive conclusions in some analyses. In addition, no data was available about when the outpatients were cleared of the infection since they did not provide swab samples for the follow-up test. Despite these limitations, this study is significant as one of the first reports on the features of confirmed pediatric COVID-19 cases in Turkey. The study presents valuable data about pediatric cases since COVID-19 is a novel disease about which data are scarce, particularly in the pediatric population. Data from more extensive multicenter studies are required to improve the diagnosis, follow-up, and treatment approaches.
In summary, this study has indicated that the main clinical features of COVID-19 in children are fever, dry cough, and mild pneumonia. Most often, the pediatric patients have been infected with SARS-CoV-2 through close contact with their family members. Pediatric COVID-19 cases have improved without any additional treatment protocols to the standard pneumonia treatments for the other known viral infections, even in hospitalized patients. Moreover, they had no sequelae until the submission of this report. Therefore, the diagnostic, clinical, and even therapeutic applications in children can be more conservative than those in adults. It might be more appropriate to consider chest CT screening, hospitalization, and the treatment options rather than supportive treatment in selected pediatric COVID-19 cases only.










 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download