Combined antiretroviral therapy (cART) is a standard treatment of human immunodeficiency virus 1 (HIV-1) infection.
12 HIV integrase is an enzyme that allows viral DNA to integrate into the genome of the host cell. Antiretroviral drug integrase strand transfer inhibitor (INSTI) has become the most preferred class of cART by virtue of rapid and potent antiviral effect and less toxicity compared to other cART drug classes.
234 Raltegravir was the first INSTI introduced in 2007, followed by elvitegravir/cobicistat/tenofovir disoproxil fumarate/emtricitabine (E/TDF/E) and dolutegravir/abacavir/lamivudine (D/A/L).
56 Besides the merits of INSTI class, elvitegravir and dolutegravir are available as single tablet regimen (STR). It is well known that lower pill burden is associated with better drug compliance which is the essential key to successful cART.
7 While INSTI based STRs have become the standard option among cART regimens for treatment-naïve HIV-1 infected individuals, it is known that INSTI may cause central nervous system adverse reaction as a kind of INSTI class effect.
2 In addition, D/A/L contains abacavir which may cause severe hypersensitivity reaction
8, and possibly cardiovascular complications.
910 Elvitegravir based STRs are required to be taken with food for better pharmacokinetics
11 and drug-drug interaction (DDI) is more common for elvitegravir based STRs due to its booster component cobicistat.
12 Relatively lower resistance barrier is another issue for elvitegravir based STRs compared to D/A/L.
13 While elvitegravir based STR is not preferentially recommended as an initial regimen anymore, it is still included in most guidelines as an alternative regimen.
24
In Korea, the first STR, E/TDF/E was introduced in 2014, followed by D/A/L in 2015 and E/tenofovir alafenamide (TAF)/E in 2017. In this study, the durability of INSTI based STR was compared between E/tenofovir/E (E/T/E, E/T/E includes E/TDF/E and E/TAF/E.) and D/A/L in cART naïve HIV-1 infected individuals for 96 weeks. The reason and incidence of discontinuation of initial STR were also reviewed.
This was a retrospective, longitudinal analysis of ART-naive, HIV-1 infected individuals enrolled from National Medical Center in Seoul, Korea. Cohort of patients starting ART with INSTI STR was constructed from electrical medical database. Demographics, HIV infection risk group, viral load, CD4+ T cell counts, cART history, and cART related adverse events (AEs) were obtained by medical record review. Eligible patients were ART- naïve HIV-1 infected individuals with age over 18 in whom ART with INSTI STR was initiated between March 1, 2014 and June 30, 2017. The interval between the start and discontinuation of STR was calculated to evaluate the durability of STR. However, change of E/TDF/E into E/TAF/E was considered as continuation of initial STR because E/TAF/E was a substitute of E/TDF/E with less bone and renal toxicities. As a result, comparison of STR was done between D/A/L and E/T/E.
As study endpoints, reasons for STR discontinuation were investigated during the 96 weeks after STR initiation. Untypable discontinuation cases were not considered, such as follow-up loss or if the reason of discontinuation was unavailable. Typable discontinuations were classified as drug adverse event related discontinuation (AEDC) and non-adverse event related discontinuation (NAEDC). NAEDCs included DDI, avoidance of food requirement with medication, and virologic failure. Virologic failure was defined as more than two successive viral load > 200 copies/mL without documented suboptimal compliance. Because STRs were introduced sequentially, their follow-up durations were different during the study period. The incidence of discontinuation was calculated as number of events per 100 person-year (PY). Incidence rate ratio (IRR) was calculated to compare discontinuation incidence between two STRs.
Finally, the durability of STR at week 96 was investigated as the interval between STR initiation and typable discontinuations. Untypable discontinuations and end of June 2018 were censored for durability evaluation.
We compared means for continuous variables by using independent Student t-tests. Categorical variables were compared by using Fisher's exact test or Pearson's χ2. Statistical comparison of discontinuation IRR was performed with 95% confidence interval (CI). Kaplan-Meier analysis including log-rank testing was used to compare durability for specific STRs with respect to typable discontinuation. Statistical significance was defined at 95% CI (two-sided). All statistical analyses were performed using SPSS version 25 (IBM Corp., Armonk, NY, USA).
During the study period, 153 started cART with D/A/L and 234 started with E/T/E (
Supplementary Table 1). In the E/T/E group, 215 started E/TDF/E and 166 switched to E/TAF/E during the study period. Nineteen started E/TAF/E from the initial. Age, sex, body mass index, HIV risk group, underlying comorbidities, cardiovascular risk factors, and baseline CD4+ cell count was not significantly different between D/A/L and E/T/E groups while median HIV-1 viral load was higher in the E/T/E group (26,858 vs. 17,658 copies/mL,
P = 0.022). Median observation duration until 96 weeks was longer in E/T/E than that in D/A/L (672 vs. 586 days,
P < 0.001). Cumulative observation duration was 228.8 PY in D/A/L group and 408.2 PY in E/T/E group.
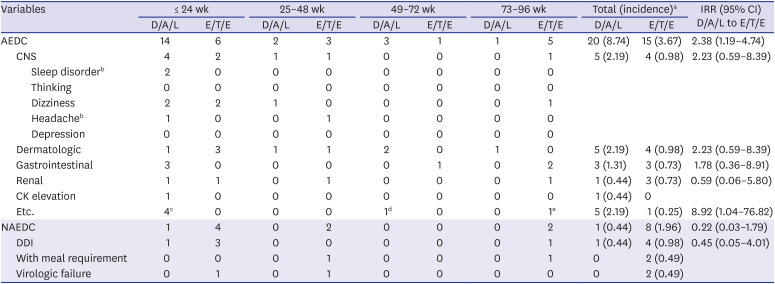
During 96 weeks, 73 individuals discontinued STR and the reason of discontinuation was typable in 44. AEDC was more common reason for discontinuation (35 vs. 9) and frequency of AEDC was higher in D/A/L group (D/A/L 20/153, 13.1% vs. E/T/E 15/234, 6.4%;
P = 0.023). Among AEDC, central nervous system (CNS) and dermatologic symptom were most common reasons (n = 9, each) followed by gastrointestinal symptom (n = 6) and renal AE (n = 4) (
Table 1). Dizziness was most common CNS AE (6/9) followed by sleep disorder (2/9) and headache (2/9). Incidence of CNS, dermatologic, and gastrointestinal AEs were higher in D/A/L group while renal AE was more common among E/T/E group. While the D/A/L to E/T/E IRR of individual AEs were not statistically significant, overall AEDC incidence of D/A/L during 96 weeks was higher than that of E/T/E (IRR, 2.38; 95% CI, 1.19–4.74). However, AEDC occurred usually within first 24 weeks (20/35, 57.1%) and incidence difference between two INSTI STR groups was significant only in this period (IRR, 3.71; 95% CI, 1.36–10.10) (
Fig. 1).
Fig. 1
AEDC incidence was higher in D/A/L group, but the difference was significant only for the first 24 weeks.
AEDC = adverse event related discontinuation, D/A/L = dolutegravir/abacavir/lamivudine, E/T/E = elvitegravir/cobicistat/ tenofovir disoproxil fumarate/emtricitabine and elvitegravir/tenofovir alafenamide/emtricitabine, PY = person-year, IRR = incidence rate ratio, CI = confidence interval.
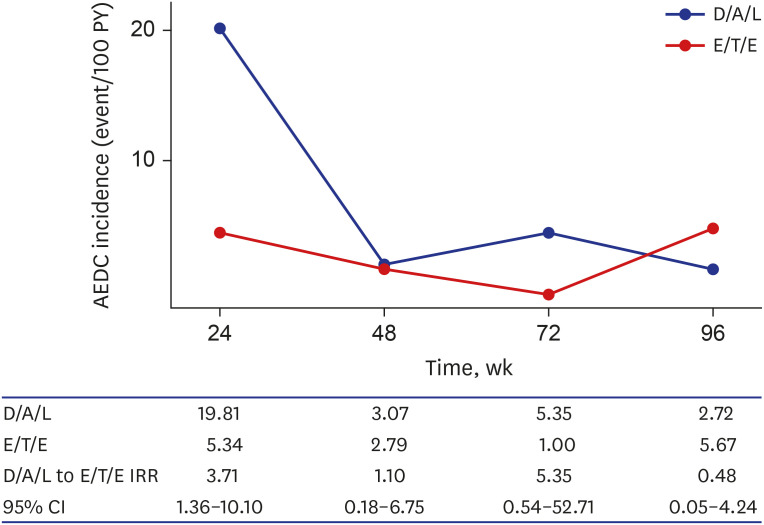

Table 1
Reasons and incidence of single tablet regimen discontinuation
|
Variables |
≤ 24 wk |
25–48 wk |
49–72 wk |
73–96 wk |
Total (incidence)a
|
IRR (95% CI) D/A/L to E/T/E |
|
D/A/L |
E/T/E |
D/A/L |
E/T/E |
D/A/L |
E/T/E |
D/A/L |
E/T/E |
D/A/L |
E/T/E |
|
AEDC |
14 |
6 |
2 |
3 |
3 |
1 |
1 |
5 |
20 (8.74) |
15 (3.67) |
2.38 (1.19–4.74) |
|
CNS |
4 |
2 |
1 |
1 |
0 |
0 |
0 |
1 |
5 (2.19) |
4 (0.98) |
2.23 (0.59–8.39) |
|
|
Sleep disorderb
|
2 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
|
Thinking |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
|
Dizziness |
2 |
2 |
1 |
0 |
0 |
0 |
0 |
1 |
|
|
Headacheb
|
1 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
|
|
Depression |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Dermatologic |
1 |
3 |
1 |
1 |
2 |
0 |
1 |
0 |
5 (2.19) |
4 (0.98) |
2.23 (0.59–8.39) |
|
Gastrointestinal |
3 |
0 |
0 |
0 |
0 |
1 |
0 |
2 |
3 (1.31) |
3 (0.73) |
1.78 (0.36–8.91) |
|
Renal |
1 |
1 |
0 |
1 |
0 |
0 |
0 |
1 |
1 (0.44) |
3 (0.73) |
0.59 (0.06–5.80) |
|
CK elevation |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 (0.44) |
0 |
|
|
Etc. |
4c
|
0 |
0 |
0 |
1d
|
0 |
0 |
1e
|
5 (2.19) |
1 (0.25) |
8.92 (1.04–76.82) |
|
NAEDC |
1 |
4 |
0 |
2 |
0 |
0 |
0 |
2 |
1 (0.44) |
8 (1.96) |
0.22 (0.03–1.79) |
|
DDI |
1 |
3 |
0 |
0 |
0 |
0 |
0 |
1 |
1 (0.44) |
4 (0.98) |
0.45 (0.05–4.01) |
|
With meal requirement |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
1 |
0 |
2 (0.49) |
|
|
Virologic failure |
0 |
1 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
2 (0.49) |
|

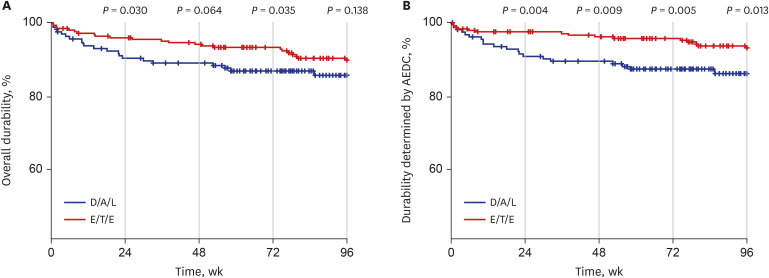
Durability assessment at week 96 was available for 358 individuals. It was not significantly different between the two groups for all typable discontinuations (
P = 0.138) (
Fig. 2A). However, worse durability related to AEDC was observed in D/A/L group at week 96 (
P = 0.013) (
Fig. 2B) and it was statistically significant throughout the study period.
Fig. 2
Durability comparison between too STRs. (A) Overall durability at week 96 was not significantly different between D/A/L and E/T/E. (B) However, worse durability related to AEDC was observed in D/A/L group at week 96 and throughout the study period.
AEDC = adverse event related discontinuation, D/A/L = dolutegravir/abacavir/lamivudine, E/T/E = elvitegravir/cobicistat/tenofovir disoproxil fumarate/emtricitabine and elvitegravir/tenofovir alafenamide/emtricitabine.

In this study, we reviewed the durability of two commonly prescribed STRs, D/A/L and E/T/E. STR is preferred by patients for convenience and lower pill burden is related to better virologic outcome.
7 While there have been several reports describing real world clinical outcomes comparing different INSTIs.
1415161718 this is the first study directly comparing D/A/L and E/T/E to the best of our knowledge. After the introduction of new INSTI Bictegravir based STR, E/T/Es are considered as alternative regimens.
24 However, E/T/Es as well as D/A/L are still one of the potent and safe STRs and many HIV-1 infected individuals are still taking E/T/Es. Therefore, our data would be beneficial to physicians and patients understanding the durability of their current INSTI based STRs and designing future regimens.
While unexpected high incidence of dolutegravir related neuropsychiatric AE has been reported in a few studies.
14151618 this has not been observed in randomized controlled trials (RCTs).
1920 While the reason of this discrepancy between RCTs and observational cohorts is unclear, higher risk of dolutegravir related neuropsychiatric AE has been reported when abacavir is combined in some studies.
1617 Higher incidence of neuropsychiatric AE related STR discontinuation in D/A/L group was also observed in this study as well as dermatologic and gastrointestinal AE, while the differences were not statistically significant. However, overall AEDC related discontinuation incidence of D/A/L was greater within the first 24 weeks and this is in line with the fact that INSTI discontinuation due to drug AE usually occurs acutely after initiation.
16 Regarding specific components associated with AEDC, one case discontinued D/A/L due to abacavir hypersensitivity reaction what was confirmed by human leukocyte antigen (HLA) typing later. AEDC due to renal function impairment occurred in three cases among E/T/E group and all of them were on E/TDF/E. While none revealed features of Fanconi's syndrome such as proteinuria or electrolyte abnormalities, renal function gradually improved after discontinuation of TDF. While AEDC was more common in D/A/L group in this study, NAEDC related discontinuation was more common in E/T/E group such as with meal requirement and virologic failure. Frequent NAEDC related discontinuation in elvitegravir treatment group was observed in another study.
16 While about half of study population were current smokers, major cardiovascular event or uncontrolled diabetes mellitus case was not observed and one case discontinued initial STR D/A/L due to hypertriglyceridemia. However, the case was enjoying frequent alcohol drinking when uncontrolled hypertriglyceridemia developed and this overall low cardio-metabolic adverse event incidence may be related with young age of study population which consists of treatment naïve HIV-1 infected individuals. Low bone mineral density, one of characteristic AE of TDF, was not observed and this also may be related with study population.
Regarding the durability of INSTI based regimen, overall durability was not significantly different between D/A/L and E/T/E at week 96 in this study. However, AEDC related durability was worse in the D/A/L group. While worse durability of dolutegravir has been reported focusing on neuropsychiatric AE,
151618 overall discontinuation rates were higher in elvitegravir than those in dolutegravir or raltegravir in one study and renal AE being alleged as a reason of the high discontinuation rate of elvitegravir,
15 It seems that renal AE and NAEDCs of E/T/E may have ameliorated the durability gap resulted from neuropsychiatric AE of D/A/L in our study.
There are some limitations in our study. Firstly, this is a retrospective study from single center and the result may not be generalizable. Secondly, among 77 discontinuation cases, the reason of STR discontinuation was available in 44 and it was not available in nine cases besides 24 follow up loss cases. Third, due to the attribute of STR, the specific component, which caused AEDC, was not identifiable in most cases and TDF/TAF were not differentiated in E/T/E group. However, no AEDC occurred among the individuals who changed from E/TDF/E to E/TAF/E.
In conclusion, AEDC occurred more frequently in D/A/L group within 24 weeks compared to E/T/E and AEDC related durability was worse at week 96 in D/A/L group. However, NAEDC was more common in E/T/E group, and overall durability at week 96 was not significantly different between D/A/L and E/T/E.
Ethics Statement
This study was approved by the Institutional Review Board (H-1807-092-006) of National Medical Center and informed consent was waived.







 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download